Griffin: Ultimate Immunoprecipitation Guide
When performing an IP experiment, consider preliminary western blots for the relevant antibodies.
RIPA (Radio ImmunoPreciptation Assay) buffer is a traditional name for an array of recipes. Below are details for optimizing immunoprecipitation experiments. Lysis buffer components (ie detergent composition, salt concentration) influence efficiency. Membrane bound protein, lipid raft, protein scaffold, and protein charge influence yield.
Cell Culture Yields
On the napkin
- Total cellular protein is lineage dependent (suspension<adherent). 5e6-10e6 cells yield ~1000 ug protein.
- 2e6 cells ~100ug(suspension) 200ug(adherent) total (RIPA) extracted
- 4e6 cells ~200ug(suspension) 400ug(adherent) total (RIPA) extracted
- ~100-300 µg cytoplasmic protein yield = 1e6 cells.
- ~1ug protein extract / 10,000-20,000 cells.
- Certain proteins (ie insoluble, modified) may escape/precipitate in classical lysis and purification procedures (ie low or no detegrent(s). Additional methods that can improve enrichment include sonication, cross-linking, and purification under denaturing conditions.
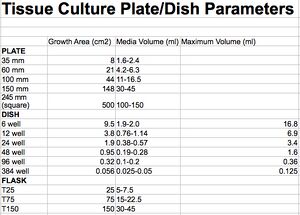
- Cell Culture Parameters
T75 Flask
- 1e6 cells ~30%
- 5e5 cells ~15%
10cm/100mm dish
- 80% confluent 10 cm dish (10ml culture media volume) / 1e6 cells can yield ~600-1000 ug total protein.
6 well/ 32-35mm
- 80% confluency (35mm / 3.5cm) of a 6-well plate contains ~8e5 and <1e6 cells / up to 300 µg cytoplasmic protein.
- 50% confluency (35mm / 3.5cm) of a 6-well plate contains ~5e5 (500K) slow growing cells.
- 50% confluency (35mm / 3.5cm) of a 6-well plate @ ~2.5e5 (250K) aggressive cells (Hela).
- (35mm / 3.5cm) of a 6-well plate culture volume @ 2-3ml.
24 well/ 12mm
- Working volume per well: 500-1000 μl
96 well/ 7mm
- Working volume per well: 50 - 335 μl
- Optimal subconfluent cell density per well: 8.0e3 (8,000) cells per well.
- 50,000 adherent cells ~70% confluency per well.
- 20,000 adherent cells ~40% confluency per well.
Solvents
- DMSO, EtOH
- <10%DMSO for in vitro drug assay
- DMSO Our analysis clearly demonstrated that DMSO cannot be considered biologically inert but induces large alterations in microRNAs (miRNA) and epigenetic landscape, especially in the maturing cardiac model.
- Optimization replicability (the same analyst re-performs the same experiment multiple times) and reproducibility (different analysts perform the same experiment using different experimental conditions) for cell-based drug screens
- methanol, ethanol and DMSO Ethanol and methanol are good choices for solvents since they have low toxicity on HepG2, MDA-MB-231, MCF-7 and VNBRCA1 cell lines. However, in the case of agents only dissolvable in DMSO, low concentrations of DMSO from 0.6%-0.015% should be considered.
Common RIPA components
Non-ionic (Zwitterionic) detergents
- Non-ionic detergents are uncharged, and containing a hydrophilic headgroup. Examples Tween, Triton, Brij. Zwitterionic (containing equal positive/negative-charge functional groups) detergents are net zero charge.
1% Nonidet P-40 or Igepal CA-630 : Non-ionic detergent to extract proteins, form lipid micelles
1% Triton X-100 : Non-ionic detergent to extract proteins, form lipid micelles - to use in place of Nonidet/Igepal
Ionic detergents
- Ionic detergents contain a head group which is either positively (cationic) or negatively (anionic) charged. Cationic detergents have a positively charged head group / quaternary ammonium group.
0.5% sodium deoxycholate (sodium salt of deoxycholic acid, anionic, bile-acid detergent) : Ionic detergent to extract membrane protein and isolate lipids. Naturally occurring Intestinal bile product deoxycholic acid mediates emulsification and absorption of fats.
0.1% SDS : Ionic detergent to extract membrane protein and isolate lipids. Anionic sodium dodecyl sulfate (SDS) carries a negatively charged sulfate group on a linear C12 hydrocarbon chain.
Physiologic salts and buffering agents
PBS : Salt prevents non-specific protein aggregation
Tris-HCl : Buffering agent prevents protein denaturation
NaCl : Buffering agent prevents protein denaturation
Phosphatase Inhibitors
Phosphorylation/dephosphorylation of proteins influences hydrostatic relationships. Proteins undergo covalent attachment of a phosphoryl group (phosphorylation) at serine, threonine, or tyrosine residues. Phosphate groups are removable via protein phosphatases. During the extraction of phosphorylated proteins from cell and tissue, there are advantages to preserving the phosphorylation states of total protein.
- (-)-p-Bromotetramisole oxalate
- Cantharidin
- Calyculin A
- Discodermia calyx
- Imidazole
- Sodium Fluoride: NaF (Sodium Fluoride) : Serine/Threonine phosphatase Inhibitor; hydrostatic interference of active sights of phosphatases
- Sodium Molybdate
- Na3VO4 (Sodium Orthovanadate) : Tyrosine phosphatase Inhibitor; hydrostatic interference of active sights of phosphatases
- Sodium Tartrate Dihydrate
Chemical Protease Inhibitors
PMSF : Stock Solution 100 mM (100x stock) or 200 mM in Isopropanol; use @ 1 mM. Store PMSF solution up to 6 months @ 4C. phenylmethylsulfonyl fluoride (PMSF) is a serine protease inhibitor binds specifically to the active site serine residue in serine hydrolases.
Protease Inhibitor Cocktails
Tableted formulations containing water-soluble protease inhibitors are user-friendly and effective substitutes in lysis buffers. When using divalent/trivalent columns for enrichment of phosphoproteins, be sure to avoid EDTA/EGTA containing cocktails.
Examples:
- S8820: Sigma SIGMAFAST™ Protease Inhibitor Tablets
- 78429: PIERCE Halt Protease Inhibitor Cocktail (100X)
- 04693116001: Complete™ Roche Protease Inhibitor Cocktail Tablets
- sc-29131: Santa Cruz Biotechnology Inc.,Protease Inhibitor Cocktail Tablet, EDTA-free
Protease Inhibitor components
- Aprotinin
- Bestatin
- Calpain Inhibitor I & II
- Chymostatin
- E-64
- Leupeptin
- Alfa-2 Macroglobulin
- Perfabloc SC
- Pepstatin
- PMSF
- TLCK-HCl
- Trypsin Inhibitor (chicken egg white, soybean)
Conjugation and Affinity Prep Kits
Protein A/G/L Agarose
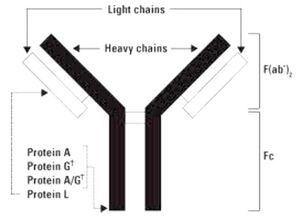
Protein A & Protein G bind to most mammalian immunoglobulins primarily through their Fc regions. Protein L is a kappa light chain specific Ig-binding protein.
Protein A/G/L are common to covalently couple by cyanogen bromide to highly cross-linked 4% agarose beads. This type of matrix is stable in most aqueous buffers.
Specifications
- Typical Ligand density: ~3 ug Protein/ul of bead
- Binding capacity: ~15-20 ug Ig/ul bead
- Bead structure: 4-6% cross-linked agarose
- Bead diameter: 40-170 um
- Temperature stability: 4-40 C
Protein A
Native protein A is a single chain (predicted 42 kDa, SDS-PAGE 46 kDa), glycoslyation-free, cell wall component produced in strains of Staphylococcus aureus. Protein A binds specifically to the Fc region of immunoglobulin molecules, including IgG. Protein A has four high-affinity (Ka = 108/mole) binding sites toward Fc region of IgG of several species (two sites can bind at a time). Protein A is heat-stable and retains conformation even after exposure to denaturing reagents such as 4 M urea, 4 M thiocyanate and 6 M guanidine hydrochloride.
Protein G
Native protein G is a bacterial cell wall protein from group G Streptococci that contains two immunoglobulin binding sites, an albumin binding site, and cell surface binding sites. Native Protein G. The recombinant form of Protein G (predicted 17-21 kDa; SDS-PAGE 31-34 kDa) contains only the two immunoglobulin binding sites to reduce nonspecific binding when purifying immunoglobulins.
Protein L
Native protein L is an kappa light chain specific Ig-binding protein that originates from the bacteria Peptostreptococcus magnus. Protein L binds Igs through interactions with the light chains. Because no part of the heavy chain is involved in the binding interaction, Protein L binds a wider range of Ig classes than Protein A or G. Protein L binds to representatives of all classes of Ig, including IgG, IgM, IgA, IgE and IgD. Single chain variable fragments (ScFv) and Fab fragments also bind to Protein L.
In humans and mice, kappa (k) light chains predominate. The remaining immunoglobulins have lambda (l) light chains. Protein L is effective in binding only certain subtypes of kappa light chains. For example, Protein L binds human VkI, VkIII and VkIV subtypes but does not bind the VkII subtype. Binding of mouse immunoglobulins is restricted to those having VkI light chains.
Protein L matrix is suitable for purification of kappa light chain-containing monoclonal antibodies from ascites or culture. Protein L is useful for purification of VLk-containing monoclonal antibodies from culture supernatant because it does not bind bovine immunoglobulins, which can be present in media. Protein L does not interfere with the antigen-binding site of the antibody.
Chicken IgY
Protein L does not react with chicken IgY light chains; PMID 15857176
Protein Quantifcation
RIPA Buffer Recipes
Commercial Lysis Buffer
Add inhibitors fresh at time of use from stock solutions
- sc-24948, 50 mL - Components supplied in four vials:
- VIAL 1: 50 mL 1X lysis buffer (pH 7.4 ±0.1) (1x PBS (sc-24946), 1% Nonidet P-40 (sc-280818), 0.5% sodium deoxycholate, 0.1% SDS)
- VIAL 2: 500 μL (200mM) PMSF in DMSO
- VIAL 3: 500 μL protease inhibitor cocktail in DMSO
- VIAL 4: 500 μL (100mM) sodium orthovanadate in water
Lysis Buffer 1:
Lysis buffer for signaling intermediates and soluble/cytosolic factors.
- 1x PBS
- 1% Nonidet P-40
- 0.5% sodium deoxycholate
- 0.1% SDS
- 1 mM Na3VO4 Sodium orthovanadate activation
- 10 mg/ml PMSF (200 mM) in isopropanol (add at 10 µl/ml RIPA)
- Protease inhibitor cocktail
Aprotinin activity is measured by KIU (KIU = Kallikrein Inhibitory Unit) Since the vial contains other components which makes the total dry. I recommend the following procedure to be used with this product: The normal working concentration range for aprotinin is either 0.5ug-2ug/ml (protein weight/volume) or 10 KIU-100 KIU/ml (units/volume). 1ug aprotinin/ml of RIPA buffer works well.
Lysis Buffer 2:
SDS free lysis buffer to consider with Co-IP; IP EGFR effectively.
- 150 mM NaCl
- 50 mM Tris-HCl pH 7.4
- 1% Nonidet P-40
- 0.25% Sodium Deoxycholate
- 1 mM EGTA
- 1mM PMSF
- Protease inhibitor cocktail
- 1 mM Na3VO4
- 1mM NaF
Lysis Buffer 3:
Brij 35 non-ionic detergent, for dissociating membrane complexes; gentler than SDS; for phospho-proteins.
- 10 mM KPO4 (phosphate buffer)
- 1 mM EDTA (chelate)
- 5 mM EGTA (chelate)
- 10 mM MgCl2 (chelate)
- 50 mM †-glycerophosphate (inhibits serine-threonine phosphotase activity)
- 0.5% NP-40 (stabilizer of proteins/enzymes)
- 0.1% Brij 35 (non-ionic detergent)
- 0.1% deoxycholic acid (non-ionic, non-denaturing detergent)
- 1 mM sodium orthovanadate (inhibits tyrosine phosphotase activity)
- Protease inhibitor cocktail
- Roux PP, Richards SA, and Blenis J. Phosphorylation of p90 ribosomal S6 kinase (RSK) regulates extracellular signal-regulated kinase docking and RSK activity. Mol Cell Biol. 2003 Jul;23(14):4796-804. DOI:10.1128/MCB.23.14.4796-4804.2003 |
Lysis Buffer 4:
RIP-seq
- 100 mM KCl
- 5 mM MgCl2
- 10 mM HEPES-NaOH pH 7.0
- 1 mM DTT
- 200 U/ml RNaseln
- 1x PIC
- 0.5% NP-40
PMID: 35973723
Lysis Buffer 5:
For IP
- 50 mM Tris (pH 7.5)
- 1 mM EDTA, 150 mM NaCl
- 1% (v/v) Triton X-100
- 1:100 (1X) Protease Inhibitor
- 1 mM sodium orthovanadate (inhibits tyrosine phosphotase activity)
- 1 mM NaF
- 5 mM MgCl2
- Benzonase (1U/million cells)
PMID: 35061527
Immunoprecipitation/Co-IP enhancement strategies
Covalent Cross-Linker
Cross linkers represent a shotgun strategy that captures interaction complexes by chemical crosslinking of intracellular proteins prior to cell lysis and immunoprecipitation.
- DSP (dithiobis(succinimidyl propionate) CAS Number 57757-57-0): Membrane-permeable, Bifunctional protein cross-linker that contains amine-reactive N-hydroxysuccinimide (NHS) esters. NHS esters react with primary amines at pH 7-9 to form covalent amide bonds. NHS-ester crosslinking reagents target primary amines of lysine (K) residues and N-terminus. Lysine residues are generally abundant and accessible on the hydrophilic phase of proteins, and cross-link with high efficiency.
- Use fresh tissue culture-grade DMSO when using a crosslinker on living cells as unsealed/opened DMSO absorbs atmospheric water, and will pre-react with the cross-linker
Detergent % Optimization
Titration of detergent composition with respect to sample type (ie cell lineage, species, tissue type) improves enrichment of lipophilic components, and improves total soluble fraction yield.
- 0.1%-1% Nonidet P-40 or Igepal CA-630 : Non-ionic detergent to extract proteins, form lipid micelles
- 0.1%-1% Triton X-100 : Non-ionic detergent to extract proteins, form lipid micelles - to use in place of Nonidet/Igepal
- 0.1%-1.5% sodium deoxycholate : Ionic detergent to extract membrane protein and isolate lipids
- 0.05%-0.1% SDS : Ionic detergent to extract membrane protein and isolate lipids
Adherent cell sample preparation
Below is a procedure for adherent cells (ie A431, A549, Hela, NIH3T3)
- Remove culture medium and rinse a subconfluent, 100 mm cell culture plate (80% confluent plate yields ~600-1000 ug protein total) with PBS at room temperature. The following steps should be performed on ice or at 4° C using fresh, ice cold buffers.
Optional: For monolayer cells, do a trypsin treatment to lift cells off the flasks prior to adding the RIPA buffer, instead of scraping the cells for a more gentle approach. If you are running a time course experiment, this is not feasible since the cells must be arrested and lysed immediately.
- Add 0.8 ml of ice cold fresh RIPA buffer to the 100 mm cell culture plates OR 0.5 ml per 5 x 10e6 cells/60 mm dish.
- Gently rock plates for 15 minutes at 4° C or let the plates set on ice. This step will allow the lysis buffer to act on the cells and will increase the total yield of soluble protein. Scrape the adherent cells with a cell scraper and then transfer the scraped lysate into a sterile microcentrifuge tube. Place the tube on ice.
Optional: wash the plate once with 0.2 ml of RIPA buffer and combine with first lysate. When running multiple plates this can be tedious and not necessary if enough attention is given to the initial harvest.
Optional: Add 10 µl of 10 mg/ml PMSF stock to the lysate. If a protease inhibtior cocktail is used fresh with the RIPA buffer, this is not necessary.
- Sonicate each sample on a 70% duty cycle or less by placing only the very tip of the pin into the vial, then slowly lowering it into the lysate until it foams completely and then stop. Alternatively, pass the lysate through a 21 gauge needle to shear the DNA & incubate 30–60 minutes on ice.
- Microcentrifuge cell lysates at 12,000xg for 15 minutes at 4°C.
- The supernatant fluid is the total cell lysate. Transfer the supernatant to a new microfuge tube and discard the pellet. Quantitate the protein amount by Bradford or BCA.
Suspension cell sample preparation
- Collect approximately 2.0 x 107 cells by low-speed centrifugation (e.g. 200xg) at room temperature for 5 minutes. Carefully remove culture medium.
- Wash the pellet with PBS at room temperature, and again collect by low-speed centrifugation. Carefully remove supernatant.
- Add 1.0 ml of ice cold RIPA buffer with freshly added (Protease Inhibitors) and/or (Phosphatase Inhibitors). Gently resuspend cells in RIPA buffer with a pipet and incubate on ice for 30 minutes.
- Further disrupt and homogenize cells by hydrodynamic shearing (21-gauge needle), dounce homogenization or sonication, taking care not to raise the temperature of the lysate. (Optional: Add 10 µl of 10 mg/ml PMSF stock; sc-3597) Incubate 30 minutes on ice.
- Transfer to microcentrifuge tube(s) and centrifuge at 12,000xg for 15 minutes at 4° C. The supernatant fluid is the total cell lysate. Transfer the supernatant to a new microfuge tube. This is your whole cell lysate. For increased protein recovery, resuspend the pellet in a small volume of RIPA, centrifuge and combine supernantants.
- The supernatant fluid is the total cell lysate. Transfer the supernatant to a new microfuge tube and discard the pellet. Quantitate the protein amount by Bradford or BCA.
Tissue extract sample preparation
There are a few approaches to optimizing protein yield from whole tissue extract. For whole animal studies, arresting the covalent modification state of the entire proteome is essential to obtaining accurate data about the treatment or phenotype effect on the tissue being extracted.
1) Immediately liquid nitrogen flash freeze tissue/organ then stored at -80 C
2) Immediately heat denature the organ from the sacrificed animal by microwave in a sealed container
- Weigh tissue and dice into very small pieces using a clean razor blade. Frozen tissue should be sliced very thinly and thawed in RIPA buffer containing (Protease Inhibitors) and/or (Phosphatase Inhibitors). Use 3 ml of ice cold RIPA buffer per gram of tissue.
- Further disrupt and homogenize tissue with a dounce homogenizer or a sonicator, maintaining temperature at 4° C throughout all procedures. (Optional: Add 30 µl of 10 mg/ml PMSF (sc-3597) stock per gram of tissue.) Incubate on ice for 30 minutes.
- Transfer to microcentrifuge tubes, centrifuge at 10,000xg for 10 minutes at 4° C. Remove supernatant and centrifuge again. The supernatant fluid is the total cell lysate. A longer centrifugation may be necessary to obtain a clear lysate.
- The supernatant fluid is the total cell lysate. Transfer the supernatant to a new microfuge tube and discard the pellet. Quantitate the protein amount by Bradford or BCA.
Immunoprecipitation Procedure
I) Incubate cell lysate (500-1000 ug) with (2-5 µg) primary antibody (optimal antibody concentration should be determined by titration) for 2 hours at 4°C.
II) Add 20 µl of appropriate agarose conjugate suspension (Protein A-Agarose, Protein G-Agarose, Protein A/G-Agarose or Protein L-Agarose).
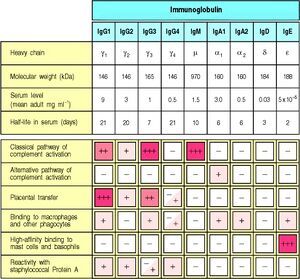
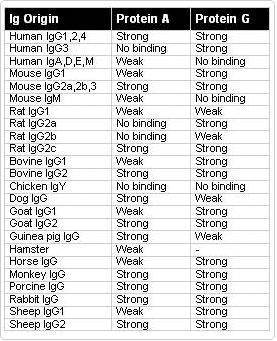
Protein A-Agarose : specific binding to mouse IgG2a, IgG2b and IgA, rabbit polyclonal Abs, human IgG1, IgG2 and IgG4
Protein G-Agarose : specific binding to mouse IgG1, IgG2a, IgG2b, IgG3, rat IgG1, IgG2a, IgG2b and IgG2c, rabbit and goat polyclonal Abs, human IgG1, IgG2, IgG3 and IgG4
Protein L-Agarose : specific binding to mouse, rat and human IgG, mouse and human IgM, IgE and IgA proteins and scFv and Fab fragments
What is Protein G PLUS? Protein G PLUS is a genetically engineered form of streptococcal Protein G that has an increased capacity and has had the albumin binding site removed to reduce background.
III) Cap tubes and incubate at 4 C on a rocker platform or rotating device for 2 hour to overnight.
IV) Collect pellet by centrifugation at 2,500 rpm (approximately 1,000xg) for 5-10 seconds. A touch spin will work. With enough samples, gravity will pellet the beads as well.
V) Carefully aspirate and discard supernatant. The trick here is to slowly aspirate with the needle touching just the top of the liquid and slowly draw down so that the needle is pulling at the surface tension of the supernatant. This will ensure no loss of beads.
VI) Wash pellet 3 times with either RIPA buffer (more stringent) or PBS (less stringent), each time repeating centrifugation step above.
VII) After final wash, aspirate and resuspend pellet in 40 µl of 2x electrophoresis sample buffer. Or elute proteins with an appropriate antibody elution buffer.
VIII) Boil samples for 2 minutes. Load sample.
Related Recipes
Electrophoresis sample buffer (2x): Mix 1.0 ml glycerol, 0.5 ml 2-mercaptoethanol, 3.0 ml 10% SDS, 1.25 ml 1.0 M Tris-HCl, pH 6.7, and 1–2 mg bromophenol blue. Store frozen in small aliquots. Alternatively, make buffer without 2 -mercaptoethanol and store at room temperature. Add 2-mercatoethanol just before using.
Sample buffer formulation:
- 7 ml Tris·Cl/SDS, pH 6.8
- 3.0 ml glycerol (30% final)
- 1 g SDS (10% final)
- 0.93 g DTT (0.6 M final)
- 1.2 mg bromphenol blue (0.012% final)
- Add H2O to 10 ml
- Store in 0.5-ml aliquots at -70°C
Latex Agglutination Assay
The latex agglutination assay is a laboratory method to check for presence of antibodies or antigens in bodily fluids;saliva, urine, cerebrospinal fluid, or blood. The test depends on what type of sample is needed.
Samples are sent to a lab, where it is mixed with latex beads coated with the specific antibody or antigen. If a affinity reactive substance is present, the latex beads will clump together (agglutinate).
For example, a child has strep throat, a throat swab is taken. The sample is mixed with latex beads that are coated with antibodies against the bacteria. The bacteria in the sample will react with the antibodies on the latex particles causing clumping (agglutination).
Latex agglutination results take about 15 minutes to an hour.