Biomod/2011/TeamJapan/Sendai/Design
<html>
<style rel="stylesheet" type="text/css">
.clear {clear:both;}
#verticalmenu {
/*this .CSS is inspired by http://www.javascriptkit.com/ */ font-family: "Comic Sans MS" , "Brush Script MT",serif, sans-serif, monospace, cursive, fantasy; list-style:none;}
- verticalmenu a:hover {
color: #aa1d1d; /* color when the click is over the main menu text-transform: uppercase; font-size: 10px; */
}
a:visited { color:#00a5ea; text-decoration: none }
.glossymenu, .glossymenu li ul{ list-style-type: none; margin: 0; padding: 0; width: 250px; /*WIDTH OF MAIN MENU ITEMS*/ border: 1px solid black; list-style:none; }
.glossymenu li{ position: relative; }
.glossymenu li a{ background: white url(http://openwetware.org/images/a/a7/Glossyback2.jpg) repeat-x bottom left; font: bold 17px Verdana, Helvetica, sans-serif; color: white; display: block; width: auto; padding: 10px 0; padding-left: 10px; text-decoration: none; }
.glossymenu li ul{ /*SUB MENU STYLE*/ position: absolute; width: 200px; /*WIDTH OF SUB MENU ITEMS*/
left: 0; top: 0; display: none; }
.glossymenu li ul li{ float: left; }
.glossymenu li ul a{ width: 190px; /*WIDTH OF SUB MENU ITEMS - 10px padding-left for A elements */ }
.glossymenu .arrowdiv{ position: absolute; right: 2px; background: transparent url(http://openwetware.org/images/6/69/Arrow.gif) no-repeat center right; }
.glossymenu li a:visited, .glossymenu li a:active{ color: white; }
.glossymenu li a:hover{ background-image: url(http://openwetware.org/images/5/50/Glossyback.jpg); }
/* Holly Hack for IE \*/
- html .glossymenu li { float: left; height: 1%; }
- html .glossymenu li a { height: 1%; }
/* End */
</style>
<script type="text/javascript">
/***********************************************
- CSS Vertical List Menu- by JavaScript Kit (www.javascriptkit.com)
- Menu interface credits: http://www.dynamicdrive.com/style/csslibrary/item/glossy-vertical-menu/
- This notice must stay intact for usage
- Visit JavaScript Kit at http://www.javascriptkit.com/ for this script and 100s more
- /
var menuids=new Array("verticalmenu") //Enter id(s) of UL menus, separated by commas var submenuoffset=-2 //Offset of submenus from main menu. Default is -2 pixels.
function createcssmenu(){ for (var i=0; i<menuids.length; i++){
var ultags=document.getElementById(menuids[i]).getElementsByTagName("ul")
for (var t=0; t<ultags.length; t++){
var spanref=document.createElement("span")
spanref.className="arrowdiv" spanref.innerHTML=" " ultags[t].parentNode.getElementsByTagName("a")[0].appendChild(spanref)
ultags[t].parentNode.onmouseover=function(){
this.getElementsByTagName("ul")[0].style.left=this.parentNode.offsetWidth+submenuoffset+"px"
this.getElementsByTagName("ul")[0].style.display="block"
}
ultags[t].parentNode.onmouseout=function(){
this.getElementsByTagName("ul")[0].style.display="none"
}
}
}
} if (window.addEventListener) window.addEventListener("load", createcssmenu, false) else if (window.attachEvent) window.attachEvent("onload", createcssmenu) </script>
|
<img src="http://openwetware.org/images/0/0a/Design.png" width="650">
|
</html> <html> <head> <style type="text/css">
- content {padding-left: 10px;width: 970px;}}
h3 {font-decoration: none;} h1.firstHeading {display: none; } </style> </head> </html>
<html> <style rel="stylesheet" type="text/css"> /*このスタイルシートの著作権はテンプレート工房TAKEにあります*/ /*ページのレイアウト用css*/
body{ background:#F5F5DC; /*壁色と壁紙設定*/ background-repeat:repeat;/*繰り返さない場合はno-repeatに変更*/ font:"メイリオ", "MS Pゴシック", Osaka, "ヒラギノ角ゴ Pro W3"; color: #333333; margin:0px; padding:0px; }
- contents{
width:900px;
margin:0 auto; background-color: #FFFFFF ;/*コンテンツ内の背景(サイズをぴったりにすること)*/ background-repeat:repeat-y; /*縦に繰り返し*/ border:solid 1px #666666;/*サイトに枠を付ける設定,色の変更可*/
position:relative;
font-size:80%;
}
/*ヘッダー部分の設定*/
- header{
background-image:url(http://openwetware.org/images/c/c4/TeamSendai-logo3.png) ;/*ヘーダー*/ background-repeat:repeat-x; /*縦に繰り返し*/ background-position:top right; height:140px; /*ヘーダーの高さ*/ }
- header p {
font-size: 26px;
color:#333333;
padding-top: 15px; padding-left: 20px; }
/*上部メニューボタンの設定*/
- navbar{
background-color:#FFFFFF;
width: 100%;
height:40px;
position:margine;
top:100px;
left:0px;
border-top:solid 1px #FFFFFF;
border-bottom:solid 1px #FFFFFF;
}
- navbar ul{
margin:0;
padding:0; list-style-type:none; font-family:Arial, Helvetica, sans-serif; font-size: 12px; line-height:40px; letter-spacing:2px; }
- navbar li{
background-color:#000099; /*上部メニューのボタンの背景*/
float:left; width:146px; /*メニューボタンの幅*/ text-align:center; padding:0; border-right:solid 1px #ffffff; }
- navbar ul a:hover{
background-color:#0033cc; /*メニューボタンにカーソルが来た時に背景*/
width:146px; /*メニューボタンの幅*/ }
- navbar a{
color:#ffffFF;/*メニューボタンの文字の色*/
display:block; }
- navbar a:hover{
color:#999999; /*メニューの文字がカーソルが来た時、この色に変わる*/ }
/*サイドメニューの設定*/
- side{
background-color:#ddffff;
width:220px;/*サイドの幅(変更するときはコンテンツ背景も変更すること)*/
position:margin; top:600px;/*上からの位置*/ left:12px; }
- side h3 {
font-size: 90%; border: double 3px #FFFFFF; color:#ffffff; text-align: center; background-color:#999999;
width:190px;
line-height: 30px; margin-top: 10px; margin-left: 5px; margin-bottom: 5px; }
- side h3 a {
color:#ffffff;
font-weight:normal; }
- side ul{
font-size:100%;
line-height:220%; /*サイドの文字と文字の行間設定*/ background-color: #ddffff; margin:0px; padding-left:10px; }
- side ul a:hover {
width:180px;
background-color: #99ffff; /*サイドのカーソルオーバー時の背景色*/ color: #999999; /*サイドのカーソルオーバー時の文字色*/ }
- side ul{
list-style-type:none;
padding-left:2px; }
- side li{
padding-left:15px; /*文字の左端からの位置*/ }
- side li a{
color:#333333;/*サイドの文字色*/
width:180px;
display:block;
}
- side .ad_list li{
background-image:none;
padding-left:0; }
/*右側メイン部分の設定*/
- main{
width:630px;
padding-top:15px;
margin-left:240px;
}
/*下部のフッター部分の設定*/
address{
font-size:80%;
font-style:normal;
text-align:center;
padding-top:5px;
}
address{
background-color:#000066;
color:#ffffff;
width:882px;
padding-bottom:10px; border:none; } address a{
color:#ff9999;
}
/*文字の設定*/ h1{ font-size:60%; letter-spacing: 2px; padding-left:10px; margin: 0px; }
h1 a{
color:#FFFFFF;
font-weight:normal; }
h2{
font-size:140%;
border-left: 10px solid #000066;
border-bottom:solid 1px #000099;/*文字の下に線を入れる設定*/
width:900px;
padding-left: 5px; color:#333333; margin-top: 15px; margin-bottom: 5px; }
h3{
font-size:120%;
border: solid 1px #111111;
color:#ffffff;
background-color:#4682B4 ; line-height: 30px; padding-left:10px; margin-top: 10px; margin-bottom: 1px; }
p{
font-size:90%;/*全体の文字サイズ*/
line-height:150%;/*全体で使う、文字と文字の行間*/
margin-left:5px;
}
p img{
float:left;
margin-top:5px; /*写真の左にスペースを空ける*/
margin-left:5px; /*写真の左にスペースを空ける*/
margin-right:10px; /:写真と文字の間隔*/ }
/*リンク文字の設定*/
a{
color:#000099;
text-decoration:none;
} a:hover { color: #FF0000;/*リンクの文字の上にマウスが来た時この色に変わる*/ text-decoration: none; }
- purple{
font-size:120%;
border: solid 1px #111111;
color:#ffffff;
background-color:#9370DB; line-height: 40px; padding-left:10px; margin-top: 10px; margin-bottom: 1px; }
h5{
font-size:120%;
border: solid 1px #111111;
color:#ffffff;
background-color:#FFA500; line-height: 30px; padding-left:10px; margin-top: 10px; margin-bottom: 1px; }
h6{
font-size:120%;
border: solid 1px #111111;
color:#ffffff;
background-color:#006400; line-height: 30px; padding-left:10px; margin-top: 10px; margin-bottom: 1px; }
- red{
font-size:120%;
border: solid 1px #111111;
color:#ffffff;
background-color:#DC143C; line-height: 40px; padding-left:10px; margin-top: 10px; margin-bottom: 1px; }
- blue{
font-size:120%;
border: solid 1px #111111;
color:#ffffff;
background-color:#191970; line-height: 40px; padding-left:10px; margin-top: 10px; margin-bottom: 1px; }
</style> </html>
Body Design
Designing the molecular robot
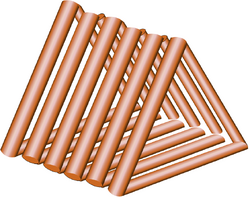
The body of our molecular robot has the shape of a triangular prism. Previous to BIOMOD2011, we have never made any DNA structure. Therefore, we thought it difficult to make a 3D structure and more than that to view it.
One of the problems in visualizing this kind of structure arise from the fact that an AFM observation is done from the top of the sample surface. So, in the case of the triangular prism we may not be able to know whether what we detect is our desire structure or not.
Under the previous circumstances, we decided to make a 2D structure: a development view taken from the structure of a triangular prism. Figure 1 and figure 2 show our 2D structure design using caDNAno and its assembled view, respectively. We planned that if both ends of the 2D structure are connected by double strands between the green and red staple (Figure 3), then, our proposed structure is complete.
Figure 3. caDNAno design: Schematic design of M13mp18 (thin line colored sky-blue) and staples
Our robot moving mechanism
We considered our robot to move along a specific direction just by rolling. Our initial plan consisted in attaching the two same legs, of three different kinds, to each edge of the triangular prism. But, our simulation predicts that we could get a better performance just by solely using one kind of legs instead of using three kinds. So, we believe that our future work would comprise the experiment of one leg type for the robot, thus, improving the robot efficiency.
Procedure for cutting DNA
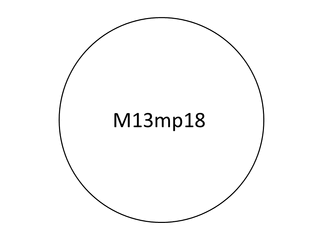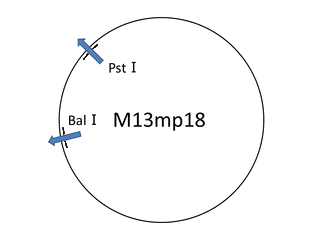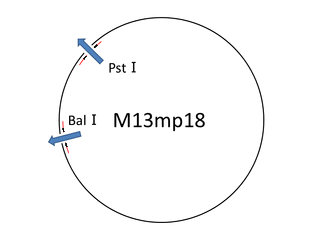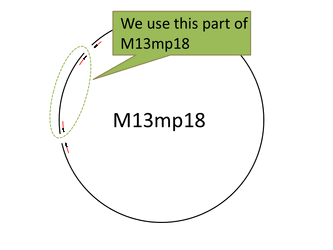
For producing the robot body we used the viral M13mp18 DNA single strand (M13) as scaffold. But we only used 1,108 bases of 7,249 bases ([1]), then having a leftover of around the 84% of the whole M13 sequence. In this situation we thought about cutting the part of M13 that we needed. Our first attempt was to extract the necessary part of M13 by reproducing M13 with polymerase chain reaction. But we failed. So we changed the method for cutting the M13 with now using restriction enzyme(Bal I and Pst I). Therefore, we found that the restriction enzyme method is an easy way to get and can cut near 1,108 bases. Then, we later successfully checked by electrophoresis whether the M13 sequence was properly cut.
Click for a detail procedure about the Method for cutting the M13.
3D DNA nanostructure
- Electrophoresis
First, we carried out electrophoresis (EP) in order to check whether the structure was correctly made. We did EP only for the M13 and did annealing for the sample mix of M13 and staples, and analyzed the difference of length between the bands. We used agarose as gel.
- Atomic force microscope
After successfully constructed the structure by EP, we observed the annealed sample by high-speed AFM. First, we observed the 2D structure. Second, as the first stage was success we proceeded to check the 3D structure.
Verification of our structure and field from the mix sample
When we carried out annealing, we added a more quantity of staples than M13s. Therefore, we tried to get rid of the over staples from sample. Because of the excess of staples, we can hardly distinguish between body structure and field.
We have three methods for removing remained staples after DNA origami folding:
- PEG (polyethylene glycol) precipitation([2])
- Freez'N squeeze ([3])
- Micro spin column by S-H400HR ([4])
These methods could remove remained staples after DNA origami folding, however it is not sure to take safely the body structure from solution.
Leg Design
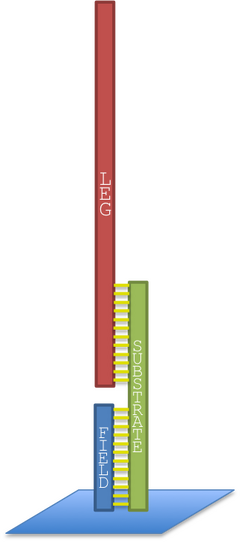
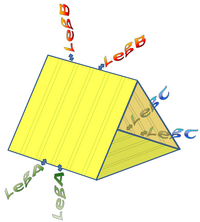
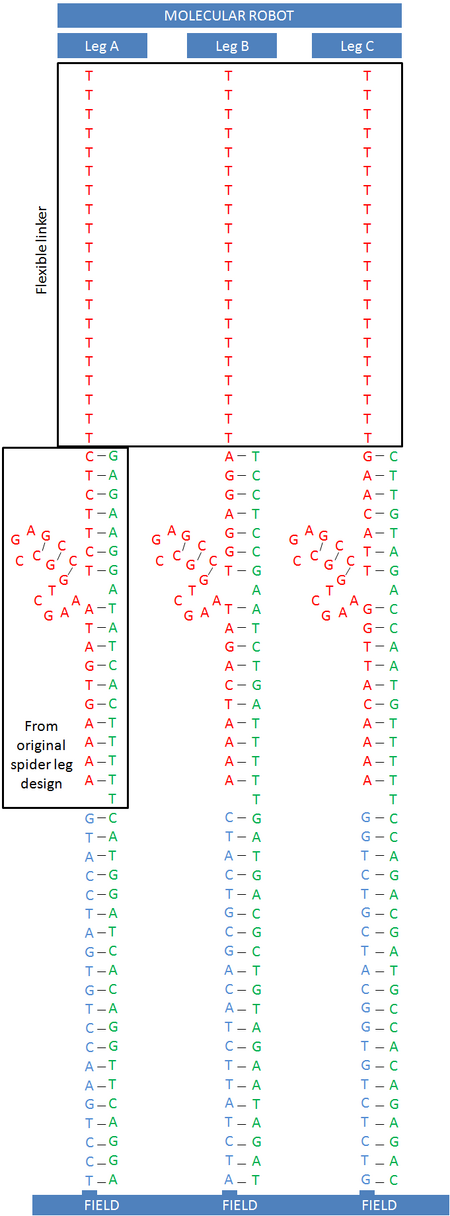
The legs of our molecular robot have certain versatility, i.e. being the same leg type or different types. In our first idea about the triangular prism robot we thought it to have three different kinds of legs for performing its rolling motion. However, our simulation program predicted the robot to have a more efficient movement by just having one type of legs [5]. In this section we would like to describe our first design where the robot utilizes three different legs, substrates and field sequences (Figure 5) for its motion mechanism. For robustness, the system is designed to have two legs of the same type in each of the three edges of the prism shape robot (Figure 6). The DNA sequences of these legs were calculated by carrying out a Monte-Carlo optimization using the Java version of DNA Design Toolbox.
Our first approach to design these three legs was based on the previous work done by Lund et al. (Nature, 2010), where they used three legs which consisted of the same sequence. For our purpose we used that sequence as the leg A (Figure 7). For leg B and C, unknown sequences have been considered.
The program needs the input of each strand and define which helices exist, from here each leg, its respective substrate foothold and the respective extended DNA sequence from the field were defined as a helix (Figure 5). These constrains help us to describe the system in such a way where there is no possibility for the legs to base pair with the robot body.
As legs should not form base pairs with the robot body we include the whole robot body sequence.
Here, we decided to include a second sequence (sticky motif robot), in addition to the prism robot sequence, as a guarantee in case that the prism robot were not successful. Thus, in that case making only the sticky motif structure with the calculated legs.
Unknown values for the nucleotides are represented as N (any base: A, C, G or T) according to the IUPAC notation.
For carrying out the Monte-Carlo optimization, it is needed to set a random number (seed). Unfortunately, there is no relation between how good the optimized sequence is and the given random number that generate it [6]. Therefore, we selected five seeds and found between them the corresponded sequence which generated the lowest best score.
We included a 'temporary' sequence defined as the sticky part and to be complementary with the base pairing common area between the substrate and leg. This sequence was used for the sake of maintaining a unique melting temperature in all the leg sequence and, consequently, being thermostable sequences. Without this 'trick' the sequence tends to form a low GC content. Then, leading to a low melting temperature (Tm). For calculating the melting temperature of those preliminary single strand DNA sequences we used the online program DINAMelt. You can download 'leg design' results by clicking here.
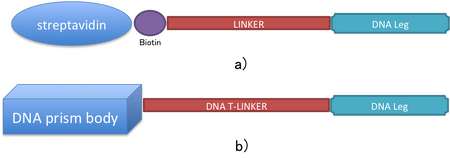
Furthermore, in order to connect the DNA body with the DNA legs we decided to use a T-linker instead of the BioTEG//iSp18//iSp18 linker [7] that binds to the streptavidin protein body. This linker is about 6nm. Therefore, the T-linker is made of 20 thymine nucleotides (Figure 8). We believe that our linker has as well flexible properties.
Location of foothold
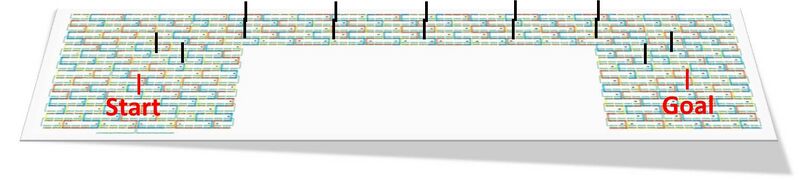

We designed the field by making a model.
Distance between each foothold lines is set to be the same length with our robot side.
At figure.5, redline are start and goal strands, and black lines are representing footholds on the field.