User talk:Sophia Helen Comas/Notebook/Biology 210 at AU
Transect # 2 Dave Mohebbi and Sophia Comas
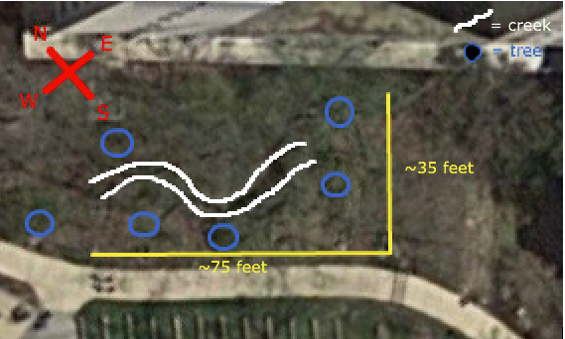
Transect # 2 is located just Northeast of the amphitheater, and consists of a moderately wooded area with a small, clear creek running through it. The land is not flat. The length of creek is the lowest point of elevation throughout the transect, with both banks rising on each side. The creek runs from the Southeast to the Northwest.
The exact boundaries of the transect area as of now unknown, as the popsicle sticks marking the boundaries were not seen.
The major biotic components of the transect are trees (marked on the map with blue circles), and grasses and bushes and small shrubbery, visible in green along the entirety of the creek. There are also many forms of wildlife, such as insects and birds, as well as lichens and algae growths on the rocks forming the creek bed. Due to the time of year, there is also an ample covering of dead leaves across most areas.
The major abiotic component of the transect is the creek itself, which runs clear and seemingly clean along the length of the transect. The creek bed is primarily stone, with rocks of many sizes and compositions, and there is a fair amount of trash and otherwise non-natural items, such as bricks, discarded food containers, and plastic bags. These are distributed relatively randomly throughout the transect.
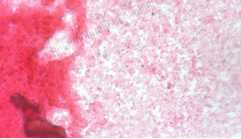
Smell & Appearance: The smell is quite horrible, with a mixture of poop and rotten milk. There is foliage on the upper most layer with small leaves and branches. This along with the grey mold on top demonstrates already that there is life in our Hay Infusion Culture. There are three large leaves on the very bottom and both the top and bottom are very dark in color when compared to the middle section, which is a little less murky.
The plant matter might differ close up vs. away because you can see the mold and foliage when you look closely at the leaves. However, farther away it is difficult to see anything other than a darkly colored leaf.
Top Niche
Picture: 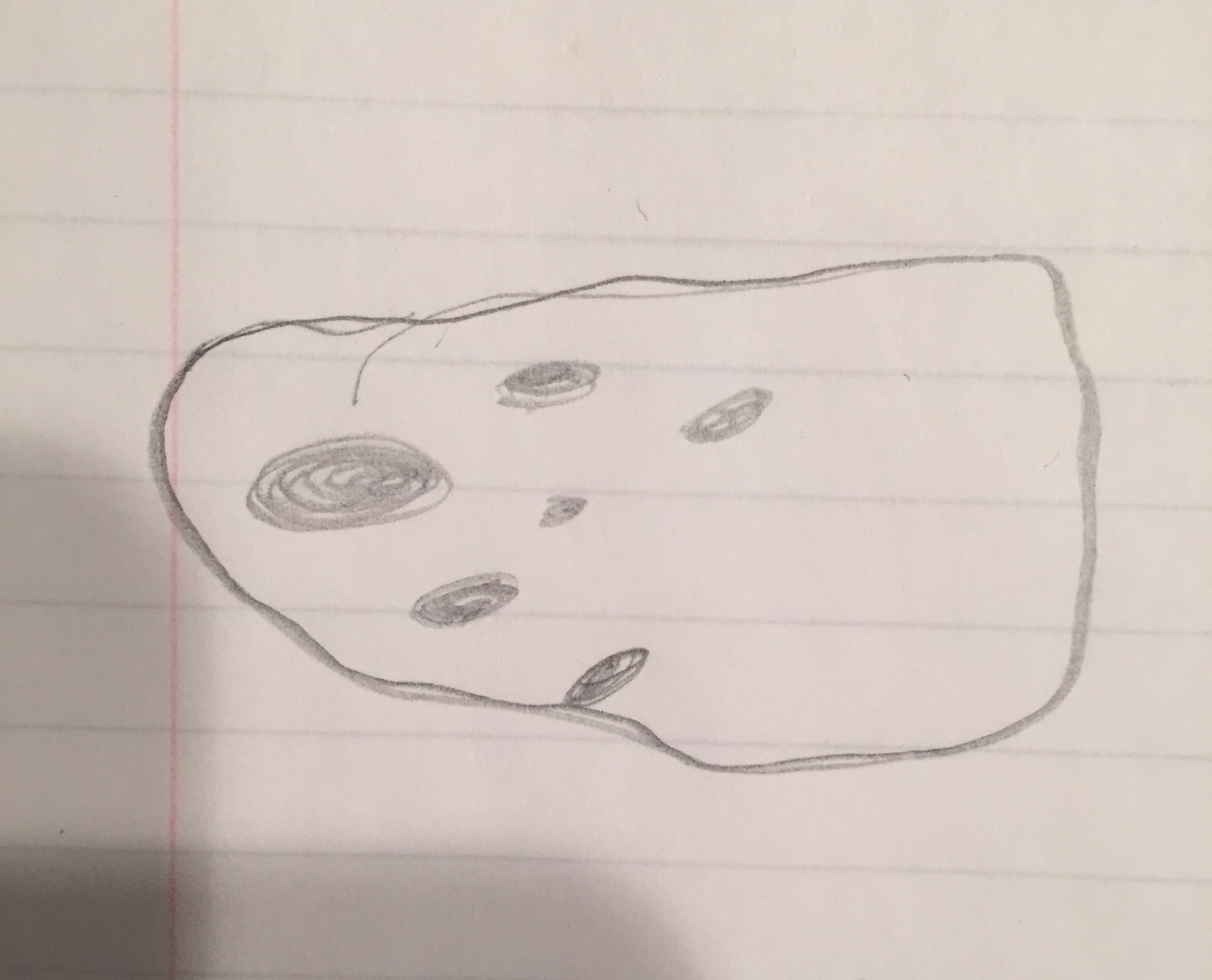 Name: Bursaria Truncatella
Motile?: Yes
Protozoa/Algae: Protozoa
Photosynthesizing?: No
Size: 40x, 17 long, 11 wide
Name: Bursaria Truncatella
Motile?: Yes
Protozoa/Algae: Protozoa
Photosynthesizing?: No
Size: 40x, 17 long, 11 wide
Picture: 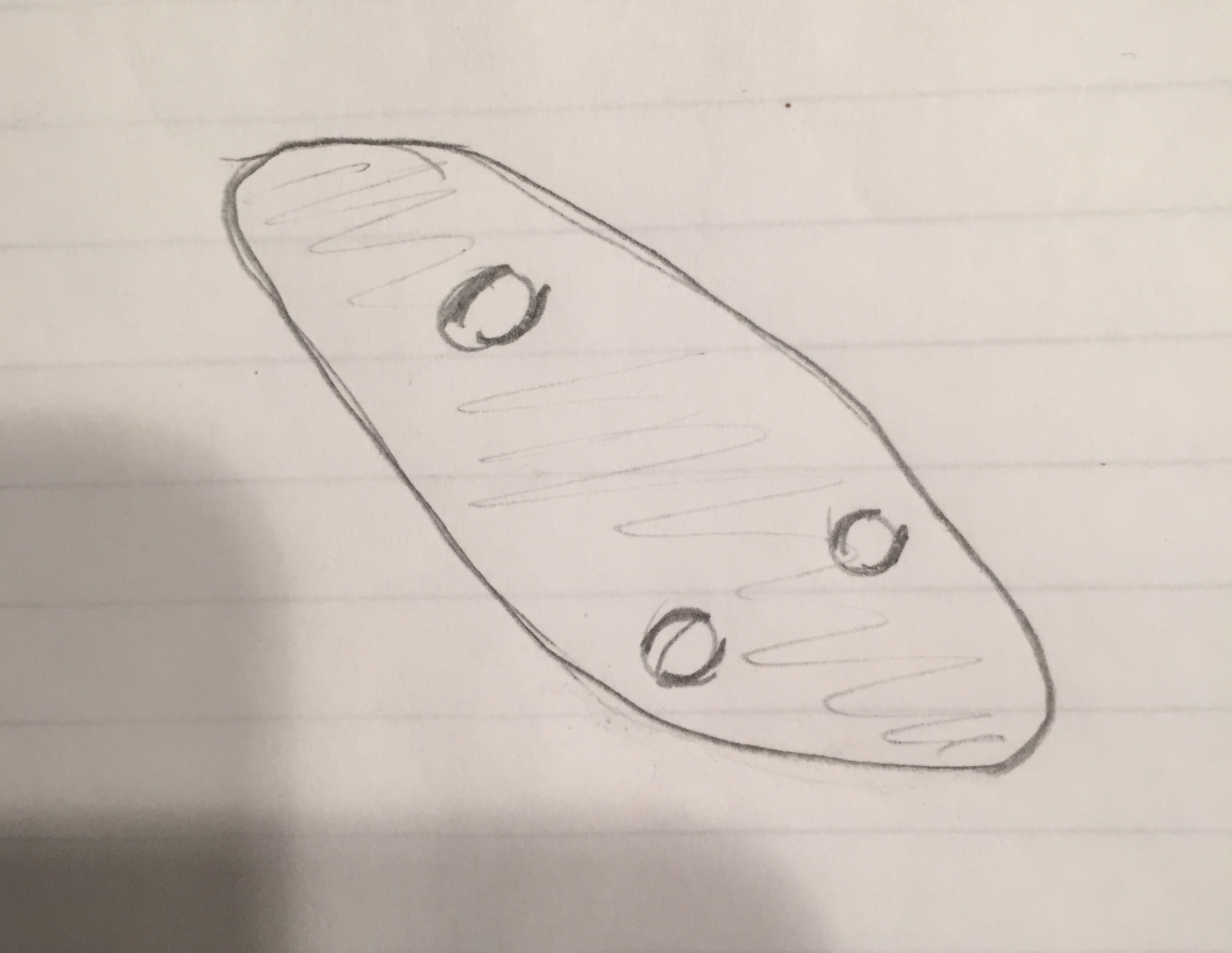 Name: Colpidium
Motile?: Yes
Protozoa/Algae: Protozoa
Photosynthesizing?: No/
Size: 40x, 4 long, 2 wide
Name: Colpidium
Motile?: Yes
Protozoa/Algae: Protozoa
Photosynthesizing?: No/
Size: 40x, 4 long, 2 wide
Middle Niche
Picture: 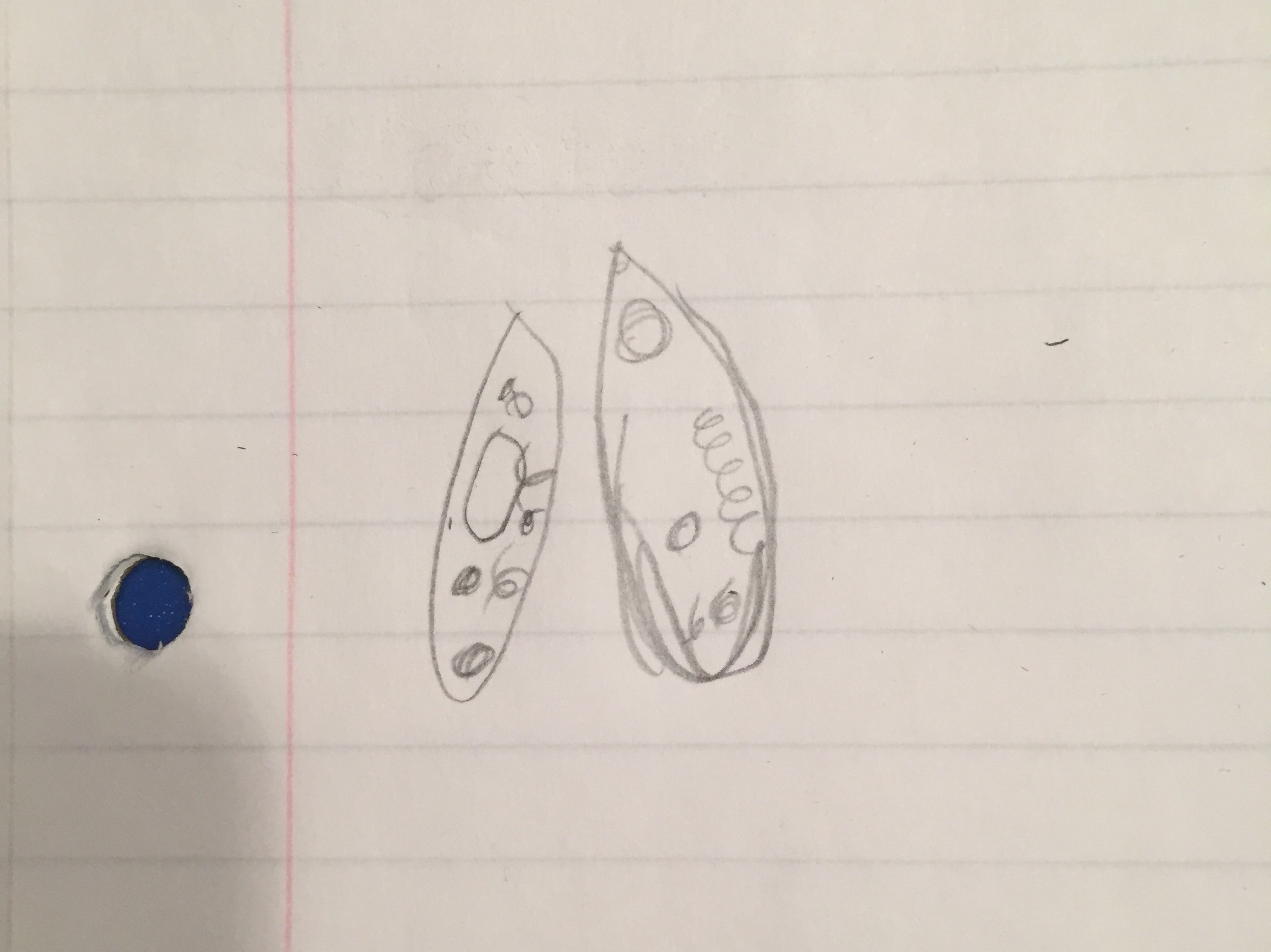 Name: Chilomonas
Motile?: Yes
Protozoa/Algae: Protozoa
Photosynthesizing?: No
Size: 40x, 5 long, 2.5 wide
Name: Chilomonas
Motile?: Yes
Protozoa/Algae: Protozoa
Photosynthesizing?: No
Size: 40x, 5 long, 2.5 wide
Picture: 
Bottom Niche
Picture: 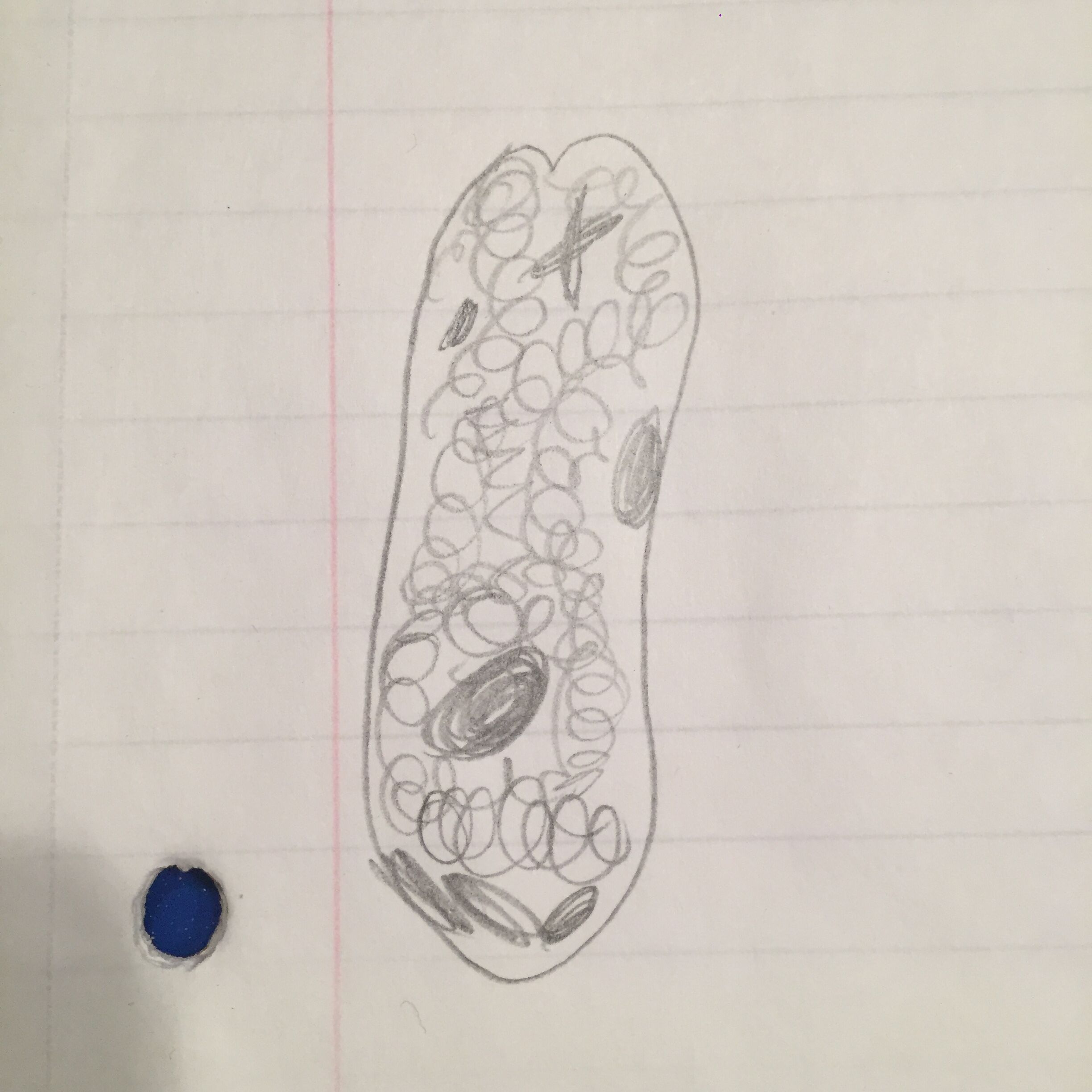
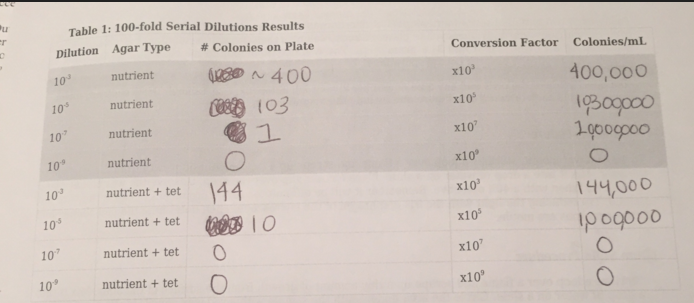
Diagram of the serial dilution procedure:
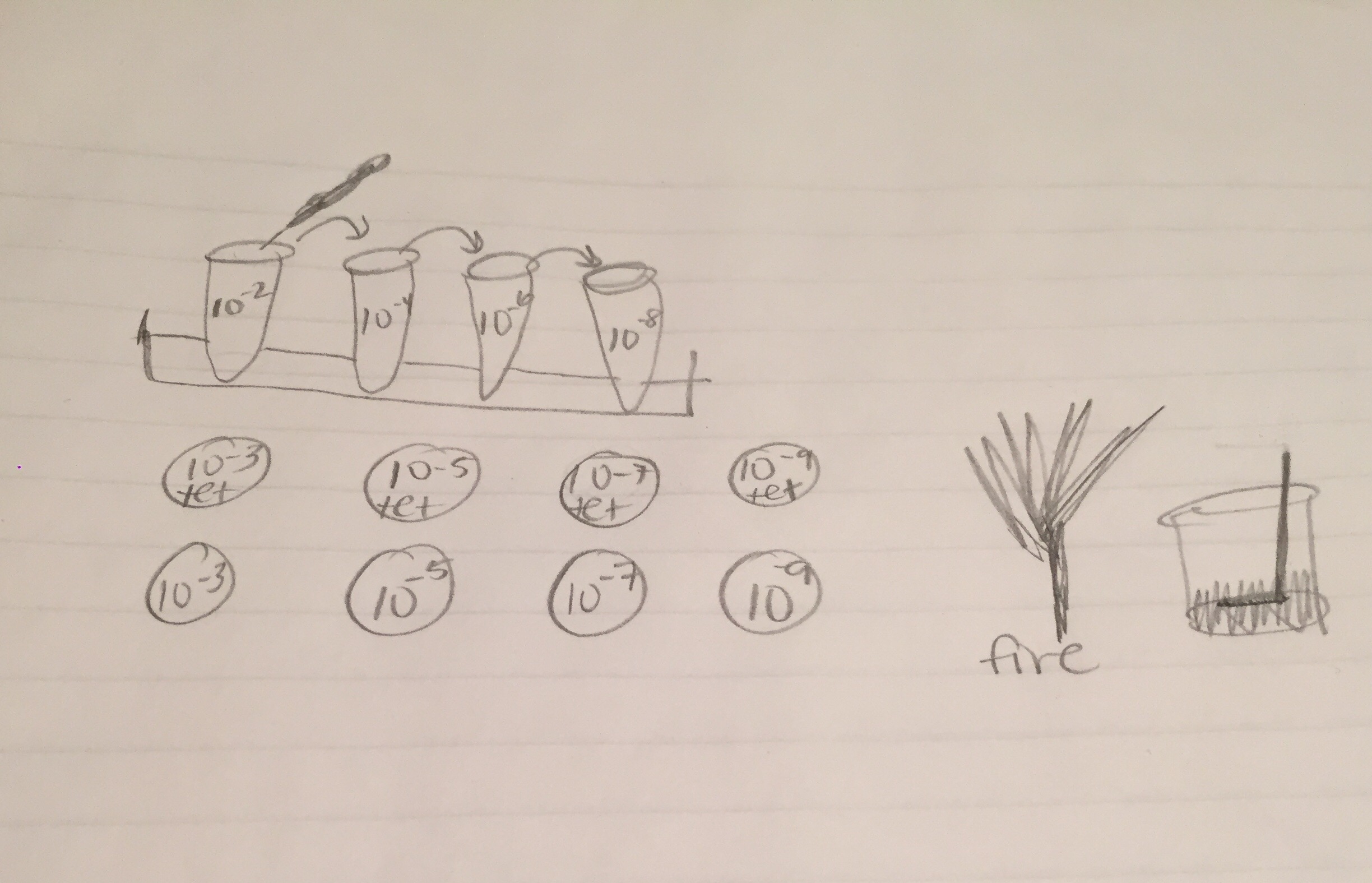
Euglena meet all the needs of life. They utilize energy, as they have photosynthesizing chloroplasts within the body of the cell, which enables them to feed by autotrophy. They have cells and genes, or information which helps them to replicate. Euglenas are a product of evolution, and each individual one will continue to replicate and share its information to future euglena.
If the Hay Infusion Culture grew for another 2 months, I would expect to see a significant increase in foliage and mold. Whereas now there are three distinct sections (top, middle, bottom), I expect that our culture would begin to blend together and the distinct sections would begin to merge together. The leaves that are so vibrantly standing out in our culture now would begin to get smaller and blend in more with the twigs and murky water. If we were to examine niches in the microscope 2 months from now, I would expect an abundance of algae and protozoa growing and possibly even other forms of life. There are several selective pressures that would affect the community of our samples. First off, it would be very still, compared to the life our sample had outdoors, with various weather patterns and wildlife to instill mobility. Our culture would remain in a plastic bottle, whereas in the outdoors it would likely move and separate various times from what’s directly surrounding each piece or water molecule. There would be nutrient scarcity seeing as this lack of mobility would limit the amount of other living organisms or species that come in contact with our hay infusion culture. Finally, the air temperature in the lab room is significantly warmer than transect 2, where there are piles of snow, rain, ice, and occasional douses of sunlight. Each of these pressures could significantly alter the community of our samples.
Final Observation of Hay Infusion Culture-
Our culture is more settled, the plant matter that was originally green is now brown and decomposed. The level of culture decreased by almost half, and the leaves and stick are significantly smaller. There is still a strong smell, and the top of the culture is now covered with a firm white film. The middle of the culture is still clear and the bottom is dark brown. I hypothesize that the appearance of the culture changed because the hay infusion is a micro ecosystem, and is moving towards equilibrium from the point that it was originally put together.
I do not expect an archaea to form on the agar plates because archaea are generally extremophiles and live in extreme environments, and not in neutral lab classrooms.
Tetracycline-
Tetracycline is a broad spectrum poly-ketide class antibiotic. Tetracycline was generally effective against almost all gram-negative and gram-positive bacteria prior to rising antibiotic resistances,. Tetracycline works by blocking the attachment of tRNA to the “A” site on the ribosomes of affected organisms, effectively blocking protein synthesis by blocking new amino acid in the chain.
(Connell, S. R., Tracz, D. M., Nierhaus, K. H., & Taylor, D. E. (2003). Ribosomal protection proteins and their mechanism of tetracycline resistance. Antimicrobial agents and chemotherapy, 47(12), 3675-3681.)
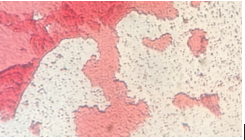
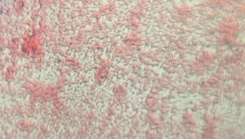
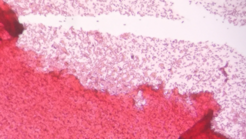
The non-tet plates had white and purple colonies, and the tet-plates had extremely vibrant red and yellow colonies. There also seemed to be much less colonies in general on the tetracycline treated plates, which makes sense, as it is an antibiotic. Due to widespread antibiotic resistances, many types of bacteria are now resistant to the effects of tetracycline. There were no noticeable fungal growths on the plates.
Cell Descriptions-
Colony Label: Tet - Red, Tetracycline Plate Plate Type: 10^-3 Colony Description: red, round, gelatinous 1-4mL Cell Description: coccus, dense Gram + or Gram - + Additional Notes: very similar
Colony Label: Non Tet - Purple, Non-Tetracycline Plate Plate Type: 10^-5 Colony Description: deep purple, waxy, gelatinous colony Cell Description: bacillus shaped Gram + or Gram - Gram Negative Additional Notes: very small cells
Colony Label:
Tet - Yellow, Tetracycline Plate
Plate Type:
10 ^-3
Colony Description:
yellow, round, gelatinous 1-4 mL
Cell Description:
coccus, dense colonies
Gram + or Gram -
+
Additional Notes:
very similar
Colony Label:
NonT - White, Non-Tetracycline Plate
Plate Type:
10^-5
Colony Description:
white, numerous, round 1-8mL
Cell Description:
coccus, dense
Gram + or Gram -
+
Additional Notes:
very small cells, staphy lococci
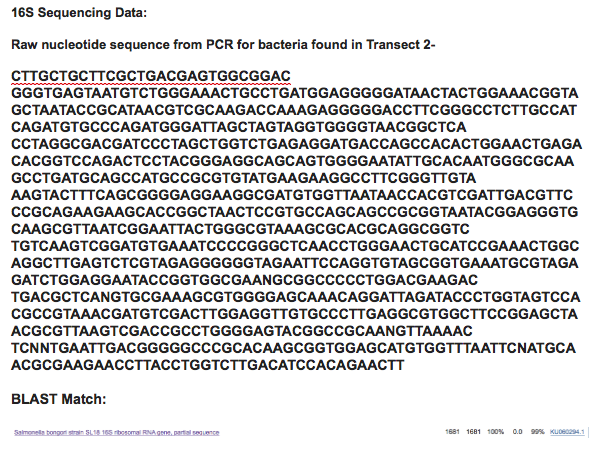
99% Match for Salmonella Bongori: Salmonella bongori is a pathogenic bacterium belonging to the genus Salmonella, and was earlier known as Salmonella subspecies V or S. enterica subsp. bongori or S. choleraesuis subsp. bongori. It is a Gram negative rod-shaped bacterium which causes a gastrointestinal disease called salmonellosis, characterized by cramping and diarrhea. It is typically considered a microbe of cold-blooded animals, unlike other members of the genus, and is most frequently association with reptiles.
Transect #2 Sophia Comas and Dave Mohebbi
Five Plants and Location in the Transect (all plants collected were vascular and therefore trachaeophyes)
Small vine growing on the creek bank close to water. Highly vascular leaves, green/purple color, wide leaves. Vascularity is easily visible from front and back of leaf. Height is about 1 inch and the tentative identification is Hedera helix, English Ivy
Tall, robust grass growing high on creek bank in large amounts. Bright gree leaves, even in the winter. Longitudinally vascular along the length of the blade. Thin blades/leaves. Tentative identification: Scirpus atrovirensHeight about 8 inches.
Thick, waxy, shrub-like grass, grows in very moist soil on creekbank near the water. Thick and spongy, longitudinally vascular along the length of the blade. Height is around 4 inches and the tentative identification is a species of Luzula, Wood rush.
Medium sized fern, grew higher on the creekbank among the bushes. Very finely vascular along the backs of the leaves. Tentative identification: Woodwardia areolata, Netted Chain Fern. Height is about 2 feet.
Relatively large bush, deep red/green/brown color, grew higher on the creekbank along the ferns, vascular along the backs of the leaves. Confident identification: Rhododendron carolinianum, Carolina rhododendron. Height about 5 feet.
No seeds found. One of the five plants collected was a fern which produces spores.
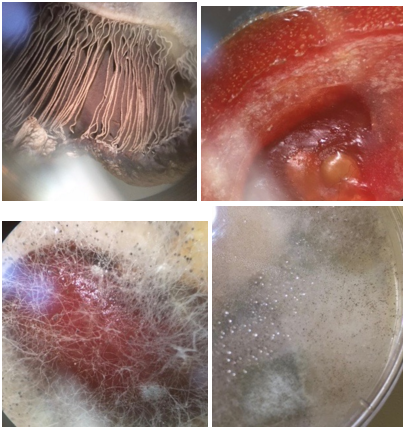
Clockwise from top left: 1) A common mushroom (saprobe) (basidiomycota) 2) Mold (rhizopus stolonifer) (saprobe) (ascomycota) 3) Mold (rhizopus stolonifer) (saprobe) (ascomycota) 4) Rhizopus Stolonifer (saprobe) (ascomycota)
Mold is a fungus where the growth of hyphae results in a discoloration and fuzzy appearance. Their network of tubular branching hyphae (a mycelium) is a single organism and is a eukaryotic micro-organism. They are decomposers of dead organic material such as leaves, wood, and plants. They also have spores.
Transect #2 Sophia Comas and Dave Mohebbi
Size range of organisms found from .1mm to 2+mm. The most common organisms were Arthropoda, Insecta, which had segmented bodies and 3 pairs of legs. The smallest organisms belonged to the class Insecta but were extremely hard to identify. The largest organisms were Arachnids with the exception of one fly (Diptera).
Arthropoda - Insecta (a fly of some sort) Features: 3 body segments, six legs, no mouth parts, two pairs of wings, compound eyes, medium sized about 1 mm in length.
Arthropoda - Arachnid Features: 8 legs in 4 pairs, abdomen and cephalothorax, very small with .25 mm.
Arthropoda - Arachnids Features: 4 pairs of legs, clearly visible abdomen and cephalothorax separation, length about 1 mm, two specimen of three arachnids found in the sample.
Arthropoda - Arachnid Features: 4 pairs of legs, cephalothorax, visible fangs, looks like a spider, length about 2mm, largest organism in the sample, one of three arachnids found.
Vertebrates:
Eastern Box Turtle (Chordata, Vertebrata, Reptilia, Testudines, Emydidae, Terrapene, T. carolina)
Beneficial Factors of Transect - insects, shrubbery (biotic), water source, shade (abiotic)
American Bullfrog (Chordata, Vertebrata, Amphibia, Anura, Ranidae, Rana, R. catesbeiana)
Beneficial Factors of Transect - insects, shrubbery (biotic), water source (abiotic)
Central Stoneroller Minnow (Chordata, Actinopterygii, Cyprinoformes, Cyprinidae, Campostoma, C. anomalum)
Beneficial Factors of Transect: insects (biotic), water source (abiotic)
Tufted Titmouse (Chordata, Aves, Passeriformes, Paridae, Baseolophus, B. bicolor)
Beneficial Factors of Transect- tress, insects, shrubbery (biotic), water source, shade (abiotic)
Red-Bellied Woodpecker) (Chordata, Aves, Piciformes, Picidae, Malanerpes, M. carolinus)
Beneficial Factors of Transect: tress, insects, shrubbery (biotic), water source, shade (abiotic).
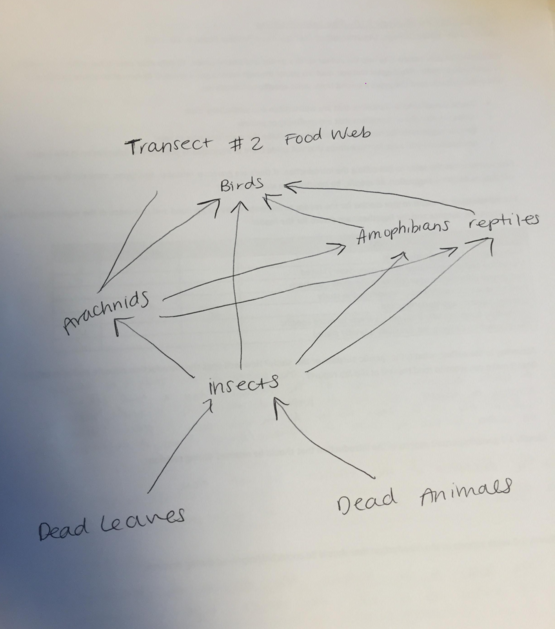
Community: A biological community consists of populations of different species that interact with one another. In our transect, the community is defined by organisms that live there and include both plants and animals. These populations interact and sustain each other as demonstrated in the food web.
Carrying Capacity: Carrying Capacity is the maximum number of individuals in a population that can be supported in a habitat over a period of time. In our transect, the carrying capacity is filled of all levels of life, and the majority is used by space, sunlight, and resultant plant life density.
Trophic Levels: Trophic levels are energy ranks in the food chain. In our transect, trophic level 1 is occupied by primary producers such as trees and grasses (autotrophs). Trophic level 2 is occupied primarily by insects, which serve as primary consumers and decomposers. Trophic level 3 feeds primarily on insects, and includes secondary consumers such as spiders, turtles, frogs, and fish. Trophic level 4 consists of tertiary consumers (and in the case of our transect, more the apex consumers) which are birds, finches, and woodpeckers.
Description of Zebrafish experiments:
Introduction: We have decided to focus our experiment on the impact of caffeine on zebrafish. We have two petri dishes, a control (20 mLs of Deerpark water) and the treated/experimental (20mLs of Deerpark water and 40mLs of caffeine). We observed that at the beginning of our experiment, the zebrafish were about 20 hours old. Hypothesis: If we put 40mLs of caffeine in the petri dish containing 20 mLs of Deerpark water and live zebrafish, then within a time span of 2 weeks, the number of alive zebrafish will decrease, the morphology will change, the size of the zebrafish will decrease, the movement will increase, the number of dead zebrafish will increase, and the heartbeat will increase.
Materials: -1 petri dish containing 24 live zebrafish and 20 mLs of Deerpark water (control)
-1 petri dish containing 24 live zebrafish and 20 mLs of Deerpark water and 40mLs of caffeine (experiment)
-A dropper
-Sharpie to label
-Dissection Scope
Methods: We filled each cell in the petri dish with 24 helathy translucent embryos and used a dropper to tansfer the eggs. We then input 20 mLs of Deerpark water of each and 40 mLs of caffeine in one of the petri dishes. We then organized our observation schedule and decided which days each of us could come in and observe, noting that Mondays and Fridays are very vital to the survival of our zebrafish.
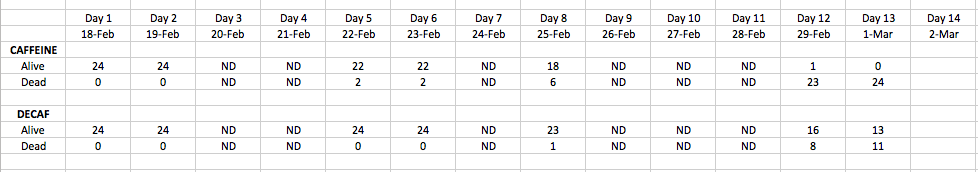
Results: Day 1 Observations (Feb 18) All eggs are healthy and about 19 hours old. All eggs are roughly the same size.
Day 2 Observations (Feb 19) Non-Caffeine Zebrafish: Embryos have continued to develop, are becoming more "fish-shaped." Tails are elongating, and some basic motility appears to be present. Not much movement or response to movement of the dish. All fish seem to be alive.
Caffeine Zebrafish: Two fish are dead. Fish are larger that non-treated zebrafish. Eyes and spots are more clearly defined than non-treated fish. Heart beat is not visible.
Day 5 Observations (2/22):
Caffeinated Zebrafish: alive in cells: 1,3,4,5,8,10,12,13,14,15,16,17,18,19,21,22,23. Embryos were larger with more defined body shapes and eyes were dark green/brown. Had one long, thin body, with two eyes that separated as branches from the body. Cells 9 and 11 were distinctly dead, as their shape was crumbled and had no form. All zebrafish were close to the edge of each cell. One clear developmental abnormality, a large bulge on the left side of the fish.
Non-caffeinated Zebrafish: alive in cells: 1,2,3,4,7,8,9,10,11,12,14,15,16,17,18,19,20,21,22,23,24.
Embryos contained the same shape and color description as those with the caffeine, however cell #17 was in the extremely early developmental stage. The zebrafish in just water also stayed by the edge of each cell. 4 more embryos were alive in this petri dish than in the caffeinated zebrafish petri dish. No clear developmental abnormality noticed.
Day 6 Observations(2/33):
Caffeinated Zebrafish: Same number of alive zebrafish. The fish are smaller, much thinner in shape, less robust, and generally less prone to movement. 1 fish growing abnormally with a bulge on his left side (see image below:)
Non-caffeinated Zebrafish: Same number of alive zebrafish. They are longer and thicker than the caffinated fish. More robust, moving around more and responsive to movement of the petri dish. There is one fish growing abnormally (see image below:)
Day 8 Observations (Feb 25): Significant differences in mortality between the caffeine and water dishes. 6 fish have died in the caffeine dish and the non-caffeinated fish are larger, more motile, and have larger eyes and darker spots. They are more responsive to stimuli and far more mobile. The eyes can be seen moving.
Day 12 Observation (Feb 29): Extreme jump in mortality in the caffeinated fish. There is only one living fish alive, with 23 dead. Decomposition seems to be happening rapidly. In the decaffeinated dish there are 16 fish still alive although seemingly much less healthy.
Day 13 Observation (March 1): All fish in the caffeine dish is dead. 3 dead in the water dish, with only 11 remaining. The fish are very unhealthy: paler, not developed in size, and almost completely unresponsive to stimuli. Once dead, the zebrafish seem to be decomposing extremely quickly. From one day to the next, there is almost no trace of zebrafish that have died.