|
Objective
- Determining the concentration of Maltose Binding Protein(MBP) solution using Bradford Assay.
Description
Preparing 1.2ml standards
- Distilled H2O was added to 1, 2, 4, 6, 8, and 10 μg/mL BSA. Approximately 200ul of Bradford reagent was pipetted into each of the six solutions.

- A blank solution containing 1ml of H2O and 200ul of Bradford reagent was prepared.
- Prepared 1/10, 1/100, and 1/1000 diluted 1ml MBP and 200ul of Bradford reagent was pipetted into each one.
- A UV-Vis Spectrophotometer (Shimadzu 2550) was used to obtain the spectra of the BSA standards, the blanks and the unknown MBP concentrations from 200nm to 800nm.
Data

A curve for the six BSA standards at 595 nm is shown below along with its best fit line. The equation was used to determine the concentration of MBP. Calculations are shown in the notes section.

- The absorbance of a 1/100 dilution of MBP at 280nm and the equation for the BSA standards at 595 nm were used to calculate the concentration of MBP.
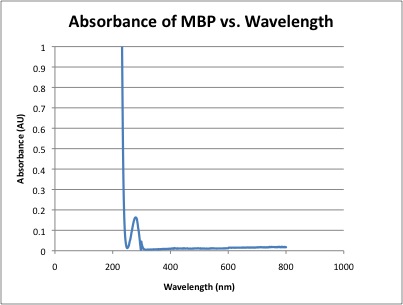
- Once the concentration of MBP is determined, the molar absorptivity of MBP could be calculated for the entire spectrum.

Notes
Calculating Molar Absorptivity of MBP:
- Corrected Absorbance of MBP at 280nm = 0.163
- Using the equation of the trend line: 0.163 = 0.0315x - 0.0205
- x = concentration of MBP x = 5.8253 ug/mL
- The MBP used was a 1/10 dilution. Therefore 58.253 ug/mL is the concentration of undiluted MBP.
- Molarity of MBP = (58.253 ug/mL) * (1000 mL / 1 L) * (1 g / 1000000 ug )* (1 mole MBP / 66776g)
- Molarity of MBP = 8.723 * 10-7 M
- Beers Law was used to calculate molar absorptivity, ε, for the entire MBP spectrum.
- A = εbc
- b= pathlength of cuvette=1cm
- Molar Absorptivity = ε = A / 8.723*10-7
- At 280nm, ε = 196,033 L / cm * mole
Use categories like tags. Change the "Course" category to the one corresponding to your course. The "Miscellaneous" tag can be used for particular experiments, as instructed by your professor. Please be sure to change or delete this tag as required so that the categories remain well organized.
.
|




