User:Anthony Salvagno/Notebook/Research/2010/01/25/Jasper PCR Again
Quick Navigation: Pages by Category | List of All Pages | Protocols
Try Number 3
Ok I discovered this morning that I just simply wasn't putting in enough primer. So before doing too much tampering, I will just add more primer. I will also raise the temp of the reaction to 64C. If this doesn't work, then I will try the 1.1kb PCR from SDM and also try a Mg++ molarity gradient. But first things first.
Gel Results
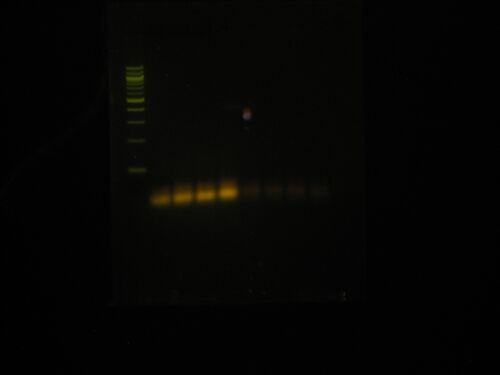
Ok this didn't work and it looks weird. I don't get why the bands are brighter in the 1st four lanes then the second four. Here is the lane designations:
- 100bp ladder
- tube 1
- tube 2
- tube 3
- tube 4
- tube a
- tube b
- tube c
- tube d
So having brighter bands means the concentrations isn't what it is supposed to be? I suppose it could also mean that Cy3 fluoresces at the wavelength of Sybr Safe, I don't know. I really have no clue what is going on.
SJK 22:43, 25 January 2010 (EST)

Well, it's at least cool that you can see the Cy3, and that you have color photo so you can discern the difference from SYBR safe. Mg++ is a bit low (1.5mM)? Sure you're using pBR322 (not pRL574?) and it's 100bp ladder? just grasping at straws. See if you can do at least 2 reactions tomorrow. Even with class, should be me and others around to stop reactions, etc. for you. I think your ideas for Diego check if Taq is a dick (1.1kb) and Mg++ are good. Definitely surprised that it didn't work. Perhaps annealing temp is too high w/ the switch ... can you see on old gel photo where the very faint bands were? Is it same location as these new bright differently colored bands?