User:Anthony Salvagno/Notebook/Research/2009/08/06/Bead Washing
Washing Beads
If you have year old beads, and are afraid of loose streptavidin binding to all your biotin without that avidin being on a bead, then you will want to follow these protocols. This will make a 250uL batch:
Koch Method
- Aliquot 50uL beads from stock and put in eppi.
- Add 950uL 1x popping buffer
- Centrifuge at 6,600g for 5 min
- discard supernatant
- Repeat steps 2-4 for a total of 3-5 times
- After discarding of supernatant, add 250ul of BGB+Popping Buffer
- Suspend 50ul beads in 950ul 1x popping buffer.
- Centrifuge at 2,200g for 15 min (change acceleration depending on bead size).
- Discard supernatant.
- Repeat steps 2-3 for a total of 3-5 times
- After discarding of supernatant, add 250ul of BGB+Popping Buffer
Destroying Clumps
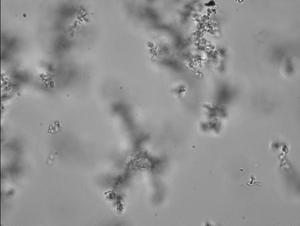
It is important to get beads to be suspended individually because clumpiness can really ruin a sample. Below are two ways that this can be achieved. With proof.
Sonication Method
You will want to sonicate beads for a decent amount of time. The time it takes depends on the size of the beads and the level of clumpiness. Also adding buffer that reduces the hydrophobicity of the bead surface may be of use.
Vortexer Method
In tests done, it seems this method works as well (or as poor depending on your perspective) as sonicating. Vortex in pulses of 5 seconds for however long you deem worthy depending on your volume and level of bead clumps.
Review
-
This image was taken after sonication.
-
This image was taken after vortexing (instead of sonicating). Seems as clumpy as sonicating.
-
This is a view of one surface of the sample in vortexing, but the same can be seen after sonication.
-
Instead of washing and sonicating/vortexing I just diluted the stock beads in water. Not dilute enough, but no clumps.
Based on these images, it seems like not washing is the best possible choice because the beads exist in solution as single entities.