User:Anthony Salvagno/Notebook/Research/2009/03/25/Miniprep and Gel
Toyoko Miniprep Protocol
Instead of using columns we did a quick protocol provided by Toyoko:
- Add 0.5ml overnight culture to 0.5ml phenol/chloroform; keep the remaining culture at 4C until we know result
- Vortex 1 minute
- Spin 12krpm for 5 min.
- Take 0.45ml top layer and mix with 0.9ml 100% cold Ethanol
- Mix and spin immediately (no need to wait 60' in this case) at 12krpm for 5 minutes
- Pour off supernatant; wash pellet with 500ul 70% Ethanol
- Dry pellet and resuspend in 20ul 1xTE plus 20ug/ml RNaseA; incubate 37C for 60 minutes
- Mix 5ul DNA prep with 3ul 5xLoading dye and 10ul H2O, save remaining DNA samples for RE digests if we ID inserts
- Load onto 1% agarose gel in TAE; Also load 250ng uncut pRS413 vector to compare
- Vectors with insert should migrate more slowly than vector control.
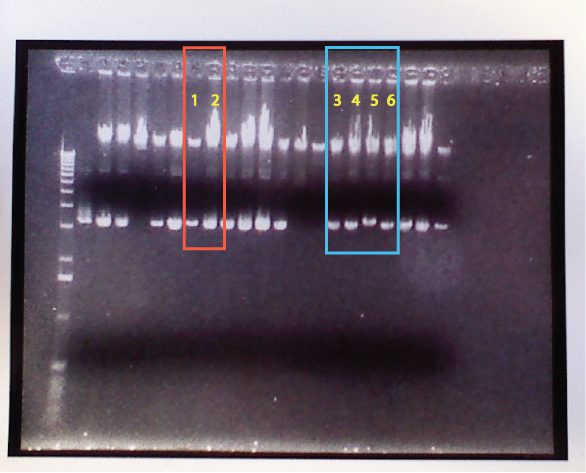
Gel Results
I didn't see anything positive. This was a major bummer. From the gel results it didn't look like we had a successful reaction, only self-ligated plasmids seemed to have been inserted. That sucks. The worst part was I prepared 20 samples and all of them failed. It looked to me like we got one or two cases that were at least worth taking a look at since I put in all the effort. We opted to go with some other samples that looked alright to Kelly. So on Friday I will work on both redoing the digest/ligation (again), and prepping the 6 colonies I chose to work from. Awesome.
Note: The 6 highlighted columns correspond to a tube of E. coli cells. I took cells from those 6 tubes and placed them into eppis labeled 1-6. These will be used in following experiments.
I hate running gels. They take so much time. It is a minimum of 2-3 hours in my experience... even the "quick" ones. Sigh.