User:Andrew K Farag/Notebook/Andrewfarag/2013/09/17
 Biomaterials Design Lab Biomaterials Design Lab
|
|
|
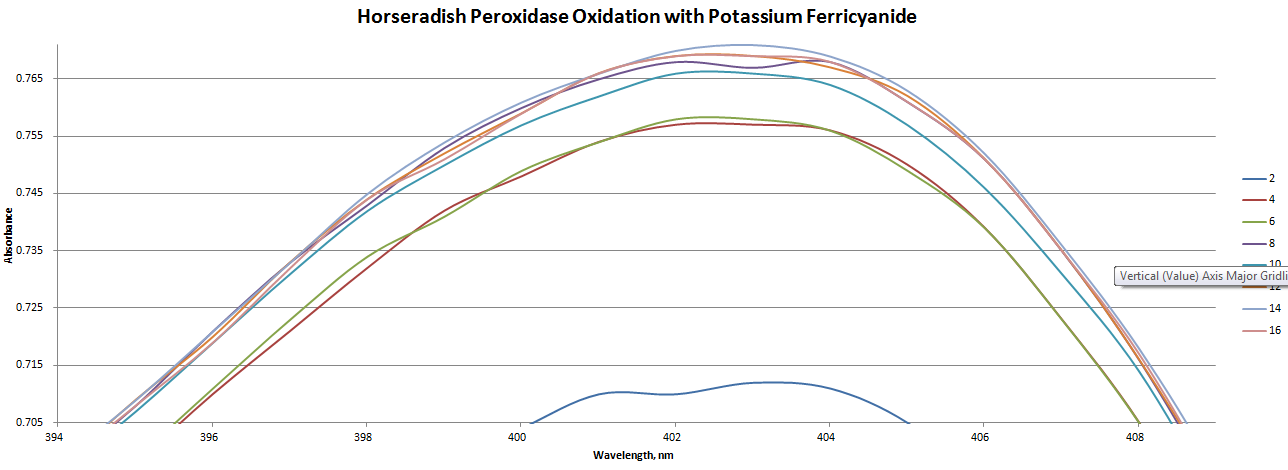
ObjectiveToday we are going to determine the amount of reagent that is required to fully oxidize or fully reduce horseradish peroxidase. For HRP oxidation, we will be using potassium ferricyanide, K3[Fe(CN)6]. K3[Fe(CN)6] has a standard reduction potential of 424mV (ref1 and ref2) vs NHE. For HRP reduction, we will be using sodium dithionite, which has a reduction potential of -460mV vs NHE. We will be monitoring oxidation and reduction through changes in the UV-Vis spectrum of HRP. In order to do this we will also have to account for the absorbance of the K3[Fe(CN)6, which has an absorption feature at 420nm (for Fe2+, ε = 4.7 M-1cm-1). This is being done in preparation for our experiments tomorrow where we will be determining the redox potential of HRP. DescriptionIn order to obtain good results, we need our buffers to be as free of oxygen (oxygen is ... an oxidizing agent, so we need to try to remove it from our experiment) as we can get them. I will prepare the buffers and reagents and will show you, group by group, how I did this. oxidation
reduction Follow along with the procedure for oxidation and, instead, use sodium dithionite for the reduction. Upon reduction the Soret peak will increase in intensity and shift to higher wavelengths. In order to prepare for tomorrow and have a better understanding of what we're doing today see the following references. This reference highlights some of the changes that we'll be observing in the spectra. Note, specifically, figure 4. We won't be using this exact experimental technique, tho. This reference goes more into detail into the kind of experiment we will be performing tomorrow. Note specifically the Redox Titrations portion of the Materials and Methods section. DataBuffer 3.0533g Tris and 1.4677g NaCl in 500mL water. pH set to 7.5 with 3M HCl Final concentration: 50.4mM Tris 50.2mM NaCl Buffer degassed by bubbling nitrogen through it for 3 hours Sodium Dithionite 19.8mg in 10mL degassed buffer --> 11.3mM 2.5mL of this solution was diluted to 25mL with degassed buffer to make a final stock concentration of 1.13mM. This solution was degassed an extra 30 minutes. K3[Fe(CN)6] 35.1mg in 10mL degassed buffer --> 10.7mM 2.5mL of this solution was diluted to 25mL with degassed buffer to make a final stock concentration of 1.07mM. This solution was degassed an extra 30 minutes. Horseradish Peroxidase 6.9mg of HRP in 10.0mL degassed buffer --> 17uM NotesThe graph of absorption vs wavelength of the Horseradish Peroxidase Oxidation with Potassium Ferricyanide is zoomed in to show a better look at the peak.
| |