|
Objective
- To run atomic absorption spectra for gold solution standards as dictated by Dr.Hartings here.<br.>
- Form a calibration curve with the class data to use for future reference.<br.>
- Calculate Concentration of Gold in Citrate Au-NP's and BSA Au-NP's.<br.>
- Calculate the number of gold particles in each sample of Citrate Au-NP's and BSA Au-NP's.<br.>
Procedure
- Samples were prepared as dictated by the table below.<br.>
 <br.> <br.>
- Please note that all samples were measured using a 100-microliter pipette and a 1000-microliter pipette.<br.>
Data
Individual and Group Calibration Curve
- The table below represents the results from the atomic absorption spectra. Please note that the max absorbance of gold was 248 nm.<br.>
- Also note that the Citrate Au-NP had to be diluted in order view on AAS. Further calculations must reflect this dilution.<br.>
 <br.> <br.>
- Absorbance had to be corrected in order to reflect the non-zero water absorbance.


- Note individual data is on the left and the class data is on the right.
Grubbs Test Analysis of Group Data
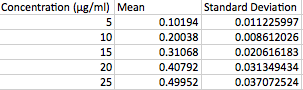
- Based on the Grubbs test, and using a value of n=5 for each concentration, no data point had a Grubbs value greater than 1.67 using 95% confidence.<br.>
- Do to this fact none of the data points are outliers.
Calculation of Concentration of Gold in Citrate Au-NP's and BSA Au-NP's
- Note that the best fit of the calibration curve is a polynomial.<br.>
- Using this best fit and the calculated absorbances of the Au-NP's the concentration of gold are the following,<br.>
- Concentration of Gold in Citrate Au-NP's = 74.45 ug/ml (This takes into account the dilution). <br.>
- Concentration of Gold in BSA Au-NP's = 38.35 ug/ml <br.>
Calculation of the # of Gold Particles in each Au-NP
- Volume of BSA Au-NP = 2.809ml
- Using the concentrations calculated using the AAS the amount of gold in the whole solution is, .00000106 moles.
- Using avogadros number their is, 6.383 * 10E17 atoms of gold in the BSA Au-NP solution
- Volume of Citrate Au-NP = 25.6 ml
- Using the concentrations calculated using the AAS the amount of gold in the whole solution is, .00000498 moles
- Using avogadros number their is, 2.999 * 10E18 atoms of gold in the citrate Au-NP solution.
- Know the concentration of the nanoparticles in solution is 8.43 nM
|
 Biomaterials Design Lab
Biomaterials Design Lab




