T7.2/Design
| Project pages on Rebuilding T7 | |
|
back to Endy Lab | |
T7.2 Alpha Design
Design
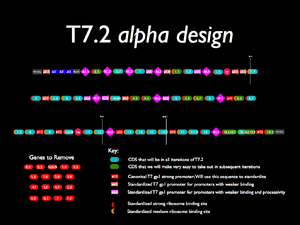
Based on the gene, RBS, RNaseIII, and promoter classifications described below, I have constructed a draft design shown in the thumbnail to the right. This is the component level specificaiton. Further refinement will be needed at the sequence level (DpnI optimization, restriction sites, codon shuffling, and how much extra DNA we will have to add).
Comments
Sri Kosuri 19:17, 28 March 2006 (EST): I'm wondering what to do when there are there are elements removed that cause two promoter elements to be right next to each other. For example, in this initial design, ø1.5 and ø1.6 (standardized to ø2.5) are right next to each other because we are planning to eliminate 1.5.
Sri Kosuri 19:35, 28 March 2006 (EST): By the definition of what coding domains we are keeping and removing (below), I think we should probably get rid of 0.3. Is there any other assignable function for this gene other than host restriction evasion?
Functional Genetic Element Design Considerations
Coding Domains
A number of the CDS on the natural T7 genome that do not contribute to phage development in the set of conditions that we wish to assay them, and thus confound our systems-level analysis. We would like to remove a number of genes which we have reason to believe encode no observable function.
The following genes are unconserved across other T7 likes and encode no known function during T7 development: 0.4, 0.5, 0.6A/B, 1.4, 1.5, 1.6, 1.8, 2.8, 3.8, 4.1, 4.2, 4.7, 5.3, 5.9, 6.3, 7, 7.7. We will begin by removing these genes first.
There is a larger subset of genes that we are interested in removing, but will continue to keep these in the genome for a variety of reasons (evolutionary conservation, contribution to development unclear). We will it make easier to delete them in the future. This subset include genes 0.3, 1.1, 4.3, 4.5, 5.5-5.7, 5.7, 6.5, 18.5, 18.7, 19.2, 19.3, 19.5.
For details on individual genes, check out the gene specification page.
Ribosome Binding Sites
We will standardize the use of Ribosome binding sites (RBS). We will assign one of these parts to each coding domain based on both knowledge of the amounts of total protein production necessary, as well as computational analysis of the existing ribosome binding sites strength. We will standardize to ribosome binding sites that were characterized by initial experiments by Heather Keller to experimentally charactize all the natural RBS strengths:
<bbpart>Z0261</bbpart> -- strong RBS -- based on gene 5 RBS
<bbpart>Z0262</bbpart> -- medium RBS -- based on BBa_B0030
For details on individual genes, check out the gene specification page.
Host Promoters
We will encode only the the 3 strong host promoters, A1, A2, and A3. We will actively take steps to remove the other host promoters identified in the annotation. Finally, we will keep the boxA anti-termination site intact. It is worth considering if we should remove all but one of the T7 promoters, such as A1.
A1,A2,A3,BoxA region -- <bbpart>Z0294</bbpart>
Removed Host Promoters
B: Located mostly in gene 0.5, which is not encoded
C: Only -35 region is included within coding domain of gene 0.7; no further mutation necessary
E: Strong -10 region mutated, -35 region not included
S1: -35 and -10 regions edited to remove promoter
S2: -35 region edited, -10 region already nonoptimal
S3: -35 and -10 regions edited to remove promoter
S4: -35 and -10 regions edited to remove promoter
S5: Located within gene 5.3 which is not included
S6: -35 and -10 regions edited to remove promoter
F1: Only -35 region is included within coding domain of gene 6.5; no further mutation necessary
F2: -35 region mutated, -10 region already nonoptimal
Phage Promoters
We want to standardize the phage promoters that we encode onto the genome. In our models, we split the promoters into three class; weak binding and processivity, weak processivity, or strong promoters. The natural T7 promoters on the T7 genome are listed below, along the classification they fall into. The promoters in bold are the canonical promoter in each class that have the most kinetic studies associated with them. Thus we will use the three promoters in bold as the prototype sequences in each class that we will standardize the other promoters to.
øOL -- weak processivity (-11,1) -- standardized to <bbpart>Z0253</bbpart>
ø1.1A -- weak binding & processivity (-17,2,4) -- standardized to <bbpart>Z0252</bbpart>
ø1.1B -- weak processivity (3,4,5) -- standardized to <bbpart>Z0253</bbpart>
ø1.3 -- weak binding & processivity (-5,5) -- standardized to <bbpart>Z0252</bbpart>
ø1.5 -- weak processivity (-2,3,4,5) -- standardized to <bbpart>Z0253</bbpart>
ø1.6 -- weak processivity (-2,3,4,5,6) -- standardized to <bbpart>Z0253</bbpart>
ø2.5 -- weak processivity (-1,1,4,5) -- defined as <bbpart>Z0253</bbpart>
ø3.8 -- weak binding & processivity (-13,-12,-11,-2) -- defined as <bbpart>Z0252</bbpart>
ø4c -- weak binding & processivity (-17,-13,2) -- standardized to <bbpart>Z0252</bbpart>
ø4.3 -- weak processivity (-2,3,4,5,6) -- standardized to <bbpart>Z0253</bbpart>
ø4.7 -- weak binding & processivity (-17,-16,-13,3,4,5,6) -- standardized to <bbpart>Z0252</bbpart>
ø6.5 -- strong -- standardized to <bbpart>Z0251</bbpart>
ø9 -- strong -- standardized to <bbpart>Z0251</bbpart>
ø10 -- strong -- defined as <bbpart>Z0251</bbpart>
ø13 -- strong -- standardized to <bbpart>Z0251</bbpart>
ø17 -- strong -- standardized to <bbpart>Z0251</bbpart>
øOR -- strong -- standardized to <bbpart>Z0251</bbpart>
Terminators
We will keep our defiinitions for the terminators as they were in T7.1. TE and Tø will remain intact, while effort will be made to not alter the CJ terminator.
TE -- <bbpart>Z0271</bbpart>
Tø -- <bbpart>Z0272</bbpart>
RNA
RNaseIII sites
We will use the R0.3 as a standard RNaseIII site for all encoded RnaseIII sites in T7.2 (except R1.1, which lies in the primary origin of replication).
R0.3 -- <bbpart>Z0273</bbpart>
Miscellaenous Elements
Terminal Repeats
Left End -- <bbpart>Z0292</bbpart>
Right End -- <bbpart>Z0293</bbpart>
Primary Origin of Replication
The primary origin of replication is located at the phi1.1A/R1.1/phi1.1B locus. In order to not disturb this critical section of the T7 genome, we will not standardize the promoters or RNAseIII site located in this section.
Primary Origin of Replication -- <bbpart>Z0291</bbpart>
Other Changes
Reporters
DpnI optimization
- Word file proposal for DpnI remapping
- PDF map of resulting restriction pattern
- Excel file containing sites on DNA to be changed
- Excel file containing graphical depiction of restriction fragments
Restriction enzyme considerations
Assemblies
Component Makeups
In Words
Left End -- Phi2.5 -- A1,A2,A3,BoxA -- R0.3 -- 0.3 -- R0.3 -- 0.7 -- R0.3 -- 1 -- ORI -- 1.1 -- 1.2 -- phi 3.8 -- R0.3 -- 1.3 -- TE -- phi2.5 -- 1.7 -- 2 -- phi2.5 -- 2.5 -- 3 -- 3.5 -- R0.3 -- phi3.8 -- 4A/B -- phi2.5 -- 4.3 -- 4.5 -- R0.3 -- phi3.8 -- 5 -- 5.5 -- 5.7 -- 6 -- R0.3 -- phi10 -- 6.5 -- 6.7 - 7.3 -- 8 -- phi10 -- 9 -- phi10 -- 10 -- Tphi -- 11 -- 12 -- R0.3 -- phi10 -- 13 -- 14 -- 15 -- 16 -- phi10 -- 17 -- 17.5 -- 18 -- R0.3 -- 18.5/18.7 -- 19/19.2/19.3 -- phi10 -- 19.5 -- Right End
In BioBrick Numbers
Z0292.Z0253.Z0294.Z0273.Z0261.Z0158.Z0273.Z0262.Z0106.Z0273.Z0262.Z0107.Z0291.Z0262. Z0108.Z0261.Z0115.Z0252.Z0273.Z0261.Z0109.Z0271.Z0253.Z0262.Z0151.Z0261.Z0116.Z0253. Z0262.Z0152.Z0262.Z0118.Z0262.Z0119.Z0273.Z0252.Z0262.Z0120.Z0253.Z0261.Z0138.Z0261. Z0139.Z0273.Z0252.Z0261.Z0121.Z0261.Z0160.Z0261.Z0154.Z0273.Z0251.Z0262.Z0159.Z0261. Z0123.Z0262.Z0124.Z0262.Z0125.Z0251.Z0262.Z0126.Z0251.Z0261.Z0127.Z0272.Z0261.Z0128. Z0262.Z0129.Z0273.Z0251.Z0262.Z0155.Z0262.Z0156.Z0262.Z0132.Z0262.Z0133.Z0251.Z0262. Z0134.Z0262.Z0157.Z0261.Z0136.Z0273.Z0261.Z0144.Z0262.Z0137.Z0251.Z0261.Z0148.Z0293
Initial Design
Initial Compilation: <bbpart>Z0501</bbpart>