Pruszak:Research: Difference between revisions
Jan Pruszak (talk | contribs) No edit summary |
Jan Pruszak (talk | contribs) No edit summary |
||
| Line 17: | Line 17: | ||
<font face="trebuchet ms" style="color:#00000"> </font> | <font face="trebuchet ms" style="color:#00000"> </font> | ||
<br> | <br> | ||
'''The goal of our work is to enhance present knowledge of lineage specification and cell-cell interactions in human neural development. ''' Our current focus is the identification of cell-contact- as well as diffusible factor-mediated determinants of neural lineage specification. | '''The goal of our work is to enhance present knowledge of lineage specification and cell-cell interactions in human neural development. ''' Our current focus is the identification of cell-contact- as well as diffusible factor-mediated determinants of neural lineage specification. | ||
We utilize human pluripotent stem cells (embryonic stem cells and induced pluripotent stem cells) as the chief model system, complemented with primary neural cell cultures, tumor cell lines and transgenic mouse model systems. Important techniques include a number of cell and tissue culture-based assays, state-of-the-art molecular and cell biology, flow cytometry and FACS, histological analysis and microscopy. | We utilize human pluripotent stem cells (embryonic stem cells and induced pluripotent stem cells) as the chief model system, complemented with primary neural cell cultures, tumor cell lines and transgenic mouse model systems. Important techniques include a number of cell and tissue culture-based assays, state-of-the-art molecular and cell biology, flow cytometry and FACS, histological analysis and microscopy. | ||
| Line 23: | Line 24: | ||
[[Image:Pruszak_pluripotent_stem.PNG|270px|thumb|right|'''Pluripotent stem cells:'''<br> Human pluripotent stem cells offer the unprecedented opportunity to study basic principles of development and embryology in a human model system. This lends itself to study self-renewal and cell proliferation on the one hand, and cell fate establishment and stabilization on the other.]] | [[Image:Pruszak_pluripotent_stem.PNG|270px|thumb|right|'''Pluripotent stem cells:'''<br> Human pluripotent stem cells offer the unprecedented opportunity to study basic principles of development and embryology in a human model system. This lends itself to study self-renewal and cell proliferation on the one hand, and cell fate establishment and stabilization on the other.]] | ||
Human pluripotent stem cells (hPSCs) represent a valuable system to study cellular processes and disease mechanisms in phenotypes of biomedical interest and to derive cells and tissues for regenerative medicine and cell therapy. Neural differentiation of human stem cells represents a promising avenue to derive specific neuronal and glial phenotypes for potential '''cell replacement''' in the therapy of nervous system disease. Among others, therapies are being investigated for degenerative neurological disorders such as Parkinson’s and Huntington’s disease, for glial disorders such as multiple sclerosis or for trauma conditions such as spinal cord injury. PSCs are, in principle, unlimited in their expansion potential and represent an epigenetically “blank slate” that enables directed patterning toward all lineages. Importantly, the differentiation of human neural cell types from human pluripotent stem cells enables the study of '''neural development''' in an understudied model organism to date only remotely accessible to biological discovery: humans.<br> | Human pluripotent stem cells (hPSCs) represent a valuable system to study cellular processes and disease mechanisms in phenotypes of biomedical interest and to derive cells and tissues for regenerative medicine and cell therapy. Neural differentiation of human stem cells represents a promising avenue to derive specific neuronal and glial phenotypes for potential '''cell replacement''' in the therapy of nervous system disease. Among others, therapies are being investigated for degenerative neurological disorders such as Parkinson’s and Huntington’s disease, for glial disorders such as multiple sclerosis or for trauma conditions such as spinal cord injury. PSCs are, in principle, unlimited in their expansion potential and represent an epigenetically “blank slate” that enables directed patterning toward all lineages. Importantly, the differentiation of human neural cell types from human pluripotent stem cells enables the study of '''neural development''' in an understudied model organism to date only remotely accessible to biological discovery: humans.<br> | ||
<br> | <br> | ||
[[Image:PSCdiff.PNG|370px|thumb|left|'''Neural differentiation of hPSCs:'''<br> Exemplifying the feasibility of using hPSCs for developmental studies, we hypothesized that factors known to neuralize tissue in embryological development could be exploited to guide PSCs toward neural fate. Indeed, we were able to induce neural differentiation by the application of the BMP antagonist Noggin, and thereby greatly enhance neuralization (here: typical neuroepithelial rosettes) as well as differentiation toward functional neurons in a dose and time-dependent manner. This illustrated that, in principle, basic aspects of neural development do apply to hPSC ''in vitro'' systems. | [[Image:PSCdiff.PNG|370px|thumb|left|'''Neural differentiation of hPSCs:'''<br> Exemplifying the feasibility of using hPSCs for developmental studies, we hypothesized that factors known to neuralize tissue in embryological development could be exploited to guide PSCs toward neural fate. Indeed, we were able to induce neural differentiation by the application of the BMP antagonist Noggin, and thereby greatly enhance neuralization (here: typical neuroepithelial rosettes) as well as differentiation toward functional neurons in a dose and time-dependent manner. This illustrated that, in principle, basic aspects of neural development do apply to hPSC ''in vitro'' systems. | ||
<br> mod. from: Pruszak & Isacson, Adv Exp Med Biol 2009]] | <br> mod. from: Pruszak & Isacson, Adv Exp Med Biol 2009]] | ||
<br>Ongoing projects in the lab aim at elucidating the role of specific '''integrin''' heterodimers in the critical transition from neural stem cell to neuroblast. In this context we aim to understand the role of context-dependent signaling pathways that control proliferation versus differentiation in the neural stem cell niche. For example, '''Hippo pathway''' components, involved in organ size regulation and cancer, have recently become a focus of study in a range of stem cell systems, but little is known about its function in mammalian neural development and its interplay with other signaling pathways. Enhanced understanding of how neural cell numbers are controlled during brain development may enable us to better control and therapeutically exploit human neural stem cell systems. | <br>Ongoing projects in the lab aim at elucidating the role of specific '''integrin''' heterodimers in the critical transition from neural stem cell to neuroblast. In this context we aim to understand the role of context-dependent signaling pathways that control proliferation versus differentiation in the neural stem cell niche. For example, '''Hippo pathway''' components, involved in organ size regulation and cancer, have recently become a focus of study in a range of stem cell systems, but little is known about its function in mammalian neural development and its interplay with other signaling pathways. Enhanced understanding of how neural cell numbers are controlled during brain development may enable us to better control and therapeutically exploit human neural stem cell systems. | ||
<br> | <br> | ||
<br> | <br> | ||
Revision as of 13:40, 14 January 2012
The goal of our work is to enhance present knowledge of lineage specification and cell-cell interactions in human neural development. Our current focus is the identification of cell-contact- as well as diffusible factor-mediated determinants of neural lineage specification.
We utilize human pluripotent stem cells (embryonic stem cells and induced pluripotent stem cells) as the chief model system, complemented with primary neural cell cultures, tumor cell lines and transgenic mouse model systems. Important techniques include a number of cell and tissue culture-based assays, state-of-the-art molecular and cell biology, flow cytometry and FACS, histological analysis and microscopy.
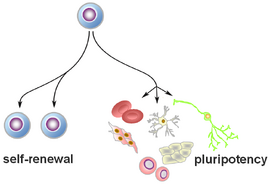
Human pluripotent stem cells offer the unprecedented opportunity to study basic principles of development and embryology in a human model system. This lends itself to study self-renewal and cell proliferation on the one hand, and cell fate establishment and stabilization on the other.
Human pluripotent stem cells (hPSCs) represent a valuable system to study cellular processes and disease mechanisms in phenotypes of biomedical interest and to derive cells and tissues for regenerative medicine and cell therapy. Neural differentiation of human stem cells represents a promising avenue to derive specific neuronal and glial phenotypes for potential cell replacement in the therapy of nervous system disease. Among others, therapies are being investigated for degenerative neurological disorders such as Parkinson’s and Huntington’s disease, for glial disorders such as multiple sclerosis or for trauma conditions such as spinal cord injury. PSCs are, in principle, unlimited in their expansion potential and represent an epigenetically “blank slate” that enables directed patterning toward all lineages. Importantly, the differentiation of human neural cell types from human pluripotent stem cells enables the study of neural development in an understudied model organism to date only remotely accessible to biological discovery: humans.
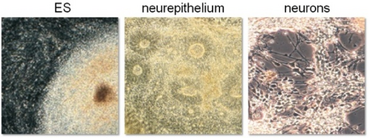
Exemplifying the feasibility of using hPSCs for developmental studies, we hypothesized that factors known to neuralize tissue in embryological development could be exploited to guide PSCs toward neural fate. Indeed, we were able to induce neural differentiation by the application of the BMP antagonist Noggin, and thereby greatly enhance neuralization (here: typical neuroepithelial rosettes) as well as differentiation toward functional neurons in a dose and time-dependent manner. This illustrated that, in principle, basic aspects of neural development do apply to hPSC in vitro systems.
mod. from: Pruszak & Isacson, Adv Exp Med Biol 2009
Ongoing projects in the lab aim at elucidating the role of specific integrin heterodimers in the critical transition from neural stem cell to neuroblast. In this context we aim to understand the role of context-dependent signaling pathways that control proliferation versus differentiation in the neural stem cell niche. For example, Hippo pathway components, involved in organ size regulation and cancer, have recently become a focus of study in a range of stem cell systems, but little is known about its function in mammalian neural development and its interplay with other signaling pathways. Enhanced understanding of how neural cell numbers are controlled during brain development may enable us to better control and therapeutically exploit human neural stem cell systems.
Selected publications:
- K. Schlegelmilch, M. Mohseni, O. Kirak, J. Pruszak, J.R. Rodriguez, D. Zhou, B.T. Kreger, V. Vasioukhin, J. Avruch, T.R. Brummelkamp, F.D. Camargo: Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 2011 144:782-95.
- J.E. Carette*, J. Pruszak*, M. Varadarajan, V.A. Blomen, S. Gokhale, F.D. Camargo, M. Wernig, R. Jaenisch, T.R. Brummelkamp: Generation of iPSCs from cultured human malignant cells. Blood 2010 115:4039-42. * equal contribution
- S. Chung, A. Leung, B.S Han, M.Y. Chang, J.I. Moon, C.H. Kim, S. Hong, J.Pruszak, O. Isacson, K.S. Kim: Wnt1-lmx1a forms a novel autoregulatory loop and controls midbrain dopaminergic differentiation synergistically with the SHH-FoxA2 pathway. Cell Stem Cell 2009 5:646-58.
- J. Pruszak, W. Ludwig, A. Blak, K. Alavian, O. Isacson: CD15, CD24 and CD29 define a surface biomarker code for neural lineage differentiation of stem cells. Stem Cells 2009 27:2928-40.
- H. Wakimoto, S. Kesari, C.J. Farrell, W.T. Curry WT, C. Zaupa, M. Aghi, T. Kuroda, A. Stemmer-Rachamimov, K. Shah, T.C. Liu, D.S. Jeyaretna, J. Debasitis, J. Pruszak, R.L. Martuza, S.D. Rabkin: Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Research 2009 69:3472-81.
- J. Pruszak, O. Isacson: Molecular and cellular determinants for generating ES-cell derived dopamine neurons for cell therapy. Adv Exp Med Biol 2009 651:112-23.
- M. Wernig, J.P. Zhao*, J. Pruszak*, E. Hedlund, D. Fu, F. Soldner, V. Broccoli, M. Constantine-Paton, O. Isacson, R. Jaenisch: Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. PNAS 2008 105:5856-61. * equal contribution
- E.M. Hedlund, J. Pruszak, T. Lardaro, W. Ludwig, A. Viñuela, K.S. Kim, O. Isacson: Embryonic stem (ES) cell-derived Pitx3-eGFP midbrain dopamine neurons survive enrichment by FACS and function in an animal model of Parkinson's Disease. Stem Cells 2008 26:1526-36.
- J. Pruszak, K.C. Sonntag, M.H. Aung, R. Sanchez-Pernaute, O. Isacson: Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations. Stem Cells 2007 25:2257-68.
- K.C. Sonntag, J. Pruszak, T. Yoshizaki, J. van Arensbergen, R. Sanchez-Pernaute, O. Isacson: Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the BMP antagonist noggin. Stem Cells 2007 25:411-8.
- S. Chung, B.S. Shin, E. Hedlund, J.Pruszak, O. Isacson et al.: Genetic selection of sox1GFP-expressing neural precursors removes residual tumorigenic pluripotent stem cells and attenuates tumor formation after transplantation. J Neurochem 2006 97:1467-80.
