BioBuilding: Synthetic Biology for Teachers: Lab 1: Difference between revisions
No edit summary |
No edit summary |
||
| Line 79: | Line 79: | ||
[[Image:Note mini.png]]''<font color = red> TEACHERS: ''If you do not have a roller wheel and an incubator, you can prepare these cultures in small erlenmeyer flasks (with stir bars) placed on a stir plate at a slow pace. Cultures are stable and active for a week at least (stored at room temp or in the fridge) but will take considerably longer to start growing on the day you subculture (~3 hours rather than 1).</font><br> | [[Image:Note mini.png]]''<font color = red> TEACHERS: ''If you do not have a roller wheel and an incubator, you can prepare these cultures in small erlenmeyer flasks (with stir bars) placed on a stir plate at a slow pace. Cultures are stable and active for a week at least (stored at room temp or in the fridge) but will take considerably longer to start growing on the day you subculture (~3 hours rather than 1).</font><br> | ||
=====Prepare turbidity standards===== | =====Prepare turbidity standards===== | ||
As the populations of bacteria increase, the culture media gets increasingly turbid. Using the [http:// | As the populations of bacteria increase, the culture media gets increasingly turbid. Using the [http://en.wikipedia.org/wiki/McFarland_standards McFarland Turbidity Scale], it is possible to estimate the changes in turbidity. The results will not be as precise as what you would measure with a spectrophotometer, but the changes over time will be detected and the results can be graphed. | ||
[[Image:McFarland_table.PNG|center]]<br> | [[Image:McFarland_table.PNG|center]]<br> | ||
[[Image:Note mini.png]]''<font color = red> TEACHERS: ''these standards can be prepared well in advance of lab and are useful if you are running the protocols without access to a spectrophotometer.</font color> | [[Image:Note mini.png]]''<font color = red> TEACHERS: ''these standards can be prepared well in advance of lab and are useful if you are running the protocols without access to a spectrophotometer.</font color> | ||
| Line 113: | Line 113: | ||
[[Image:Note mini.png]]''<font color = red> TEACHERS: ''If the entire growth curve (i.e. days 3, 4 and 5) is to be done in one class, you may have to start the early time points in advance. If you are dividing the growth curve into several short lab periods, be sure to store the cells in the fridge (~4°) until the next session. </font><br> | [[Image:Note mini.png]]''<font color = red> TEACHERS: ''If the entire growth curve (i.e. days 3, 4 and 5) is to be done in one class, you may have to start the early time points in advance. If you are dividing the growth curve into several short lab periods, be sure to store the cells in the fridge (~4°) until the next session. </font><br> | ||
=====Procedure, if no spectrophotometer is available===== | =====Procedure, if no spectrophotometer is available===== | ||
The turbidity of the bacterial populations can be estimated using the [http:// | The turbidity of the bacterial populations can be estimated using the [http://en.wikipedia.org/wiki/McFarland_standards McFarland Turbidity Scale]. This method uses suspensions of a 1% BaCl<sub>2</sub> in 1% H<sub>2</sub>SO<sub>4</sub> that are visually similar to suspensions of various populations of ''E. coli.''<br>[[Image:Turbidity_photo.jpg|thumb|center|400px| Turbidity comparisons for some bacterial cultures (left) and McFarland standards (right)]]<br style="clear:both" /> | ||
1. Following your teacher's instructions, obtain small clear test tubes containing the turbidity standards. The tubes should contain enough standard in each to fill the tube to a height of about 1 inch (2.5 cm) from the bottom. Make sure each tube is properly labeled with its turbidity standard number. If you are filling the tubes from stock bottles of the standards, use small tubes and place enough standard in each to fill the tube to a height of about 1 inch (2.5 cm) from the bottom.<br> | 1. Following your teacher's instructions, obtain small clear test tubes containing the turbidity standards. The tubes should contain enough standard in each to fill the tube to a height of about 1 inch (2.5 cm) from the bottom. Make sure each tube is properly labeled with its turbidity standard number. If you are filling the tubes from stock bottles of the standards, use small tubes and place enough standard in each to fill the tube to a height of about 1 inch (2.5 cm) from the bottom.<br> | ||
[[Image:Note mini.png]]''<font color = red> TEACHERS: ''The size of the tubes and the volume of sample and standard used is flexible. The important things are that the volume can obscure the thick black lines and that the samples and standards are prepared in the same fashion, as shown in the photo. </font color><br> | [[Image:Note mini.png]]''<font color = red> TEACHERS: ''The size of the tubes and the volume of sample and standard used is flexible. The important things are that the volume can obscure the thick black lines and that the samples and standards are prepared in the same fashion, as shown in the photo. </font color><br> | ||
Revision as of 19:51, 25 August 2010
|
Eau That Smell Lab notes |
Teacher ConsiderationsThis lab provides a valuable opportunity to teach microbiology techniques, population growth dynamics, molecular genetics and basic synthetic biology concepts in a meaningful, real world way. As can be seen in the discussion questions for the lab report, the analysis of the lab will provide the students with a chance to do meaningful error analysis and examine the difference between quantitative results and qualitative results. If time allows the students can carry out all parts of the procedure. This will take about 5 class periods in a typical High School AP Biology or Biotechnology class. If instead the teacher prepares in advance the samples as outlined in Part 1 of the procedure, the students can conduct the smell tests and population measurements over two or three days. In a college lab course, most of the bacterial growth curve can be conducted in a typical three to four hour lab period. The procedure includes instructions for using a spectrophotometer to measure the population growth. If a spectrophotometer is not available, the population can be easily measured using the McFarland Turbidity methodology, as explained below. Needed MaterialsTeacher Provides
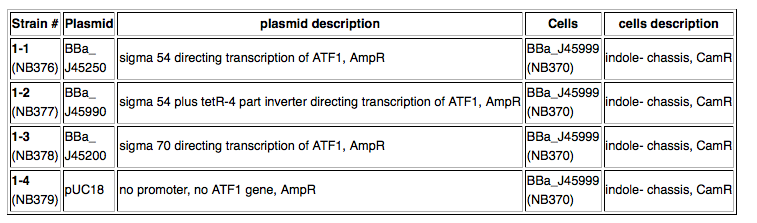
Kit Provides4 strains (see table below)
Chemicals Room Temperature
4° (fridge)
Chemical Hood
WorkflowClassroom Content
Annotated ProcedureDay 1:TO DO
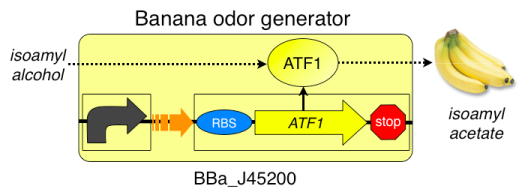
Streak out strains from stabs to platesWe will be receiving our bacteria with the plasmid already inserted. This culture will come in the form of a "stab" or "slant", a test tube with a small amount of bacteria on a slanted media. To continue the experiment we will have to further culture the bacteria by streaking out the stabs onto LB+amp plates. The plates will be incubated 37° overnight.
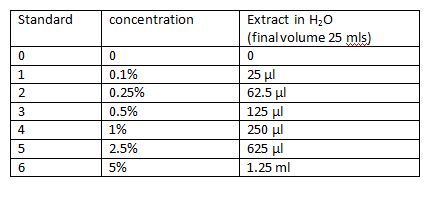
This video illustrates the technique used for this transfer. Prepare banana extract standardsThe banana extract is provided in the kit. It will be necessary to make up the standards following the table. Day 2:TO DO:
Grow liquid overnights of bacterial strains1. Using a sterile inoculating loop, transfer a bacterial colony from one of the petri dishes to a large sterile culture tube containing 5 ml of Luria Broth and 5 μl of ampicillin. Prepare turbidity standardsAs the populations of bacteria increase, the culture media gets increasingly turbid. Using the McFarland Turbidity Scale, it is possible to estimate the changes in turbidity. The results will not be as precise as what you would measure with a spectrophotometer, but the changes over time will be detected and the results can be graphed. 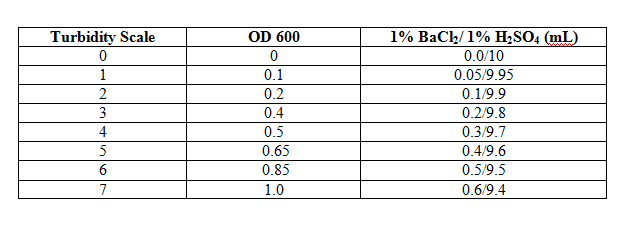
Day 3: Measuring bacterial population growth in lag phaseTO DO:
Procedure, if using a spectrophotometer1. Prepare a stock growth solution with
Procedure, if no spectrophotometer is available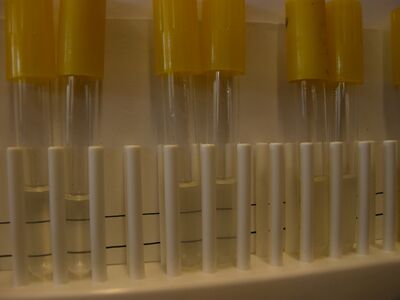 1. Following your teacher's instructions, obtain small clear test tubes containing the turbidity standards. The tubes should contain enough standard in each to fill the tube to a height of about 1 inch (2.5 cm) from the bottom. Make sure each tube is properly labeled with its turbidity standard number. If you are filling the tubes from stock bottles of the standards, use small tubes and place enough standard in each to fill the tube to a height of about 1 inch (2.5 cm) from the bottom. Day 4: Measuring bacterial population growth in log phaseTO DO:Restart cultures and collect data for lag phase
Day 5: Measuring bacterial population growth in stationary phaseTO DO:Collect data for stationary phase
AssessmentLab Report RubricLab Report ScoreSheetSurvey Monkey LinkTo help us improve the labs, you can send the students here where they can offer anonymous feedback. Thanks! Variations to try
FeedbackWe're always looking to hear back from you if you've thought about this unit, tried it, or stumbled across it and want to know more. Please email us through BioBuilder, info AT biobuilder DOT org. Navigation
|