BISC 219/2009: Mod 1 C. elegans General Information: Difference between revisions
Tucker Crum (talk | contribs) No edit summary |
Tucker Crum (talk | contribs) |
||
| Line 30: | Line 30: | ||
== Nematode Genetics == | == Nematode Genetics == | ||
The small size of ''C. elegans'' (1mm), its short generation time (3 1/2 days at 20°C), | The molecular genetics of ''C. elegans'' is well developed. This nematode holds the distinction of being the first metazoan to have its genome sequenced. In addition, most of the tricks of the molecular geneticist have been employed in ''C. elegans'', including making transgenics (including knockouts) and the technique of RNA interference: RNAi. The small size of ''C. elegans'' (1mm), its short generation time (3 1/2 days at 20°C), as well as the large number of progeny produced per animal (250-350) are also important factors in genetic analysis. Since genetic experiments frequently require the detection of rare events, it is an advantage to grow a large number of individuals in a small space. A petri plate seeded with a single hermaphrodite will contain nearly 10<sup>5</sup> individuals after one week. Confining the animals to the 2-dimensional agar surface permits the observation of rare individuals in large populations with the aid of a dissecting microscope. | ||
Self-fertilization drives populations to homozygosity so that it is easy to isolate isogenic (genetically identical) clones of animals. This greatly facilitates the detection of mutants in a diploid organism. Since chemically induced mutations first appear in the heterozygous condition, recessive mutations cannot be recognized in the F1 progeny of mutagenized animals. In ''C. elegans'' heterozygous hermaphrodites automatically segregate homozygous mutants as one fourth of their progeny, compared to “male/female” organisms where segregation of homozygous mutants requires manual cloning (brother-sister matings) and homozygotes only appear in the third generation after mutagenesis. | Self-fertilization drives populations to homozygosity so that it is easy to isolate isogenic (genetically identical) clones of animals. This greatly facilitates the detection of mutants in a diploid organism. Since chemically induced mutations first appear in the heterozygous condition, recessive mutations cannot be recognized in the F1 progeny of mutagenized animals. In ''C. elegans'', heterozygous hermaphrodites automatically segregate homozygous mutants as one fourth of their progeny, compared to “male/female” organisms where segregation of homozygous mutants requires manual cloning (brother-sister matings) and where homozygotes only appear in the third generation after mutagenesis. Additionally, ''C. elegans'' hermaphrodites may be grown and screened for mutants together, since there is no danger of losing the homozygous form of a mutant by cross-fertilization. It is also advantageous that self-fertilization does not require copulation, allowing severely uncoordinated or deformed mutants to be propagated as homozygotes. Thus, many mutations that would be lethal in ''Drosophila'' or in the mouse are viable in ''C. elegans''. This not only simplifies maintenance of genetic stocks, but it makes possible the growth of large populations of such mutants for biochemical analysis. | ||
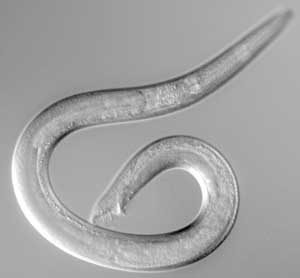
Reproduction by self-fertilization, though convenient for mutant isolation and maintenance, does not provide a means to recombine independently isolated mutations. Genetic analysis therefore depends on the existence of males, which are produced spontaneously in hermaphrodite populations by meiotic non-disjunction at a frequency of 0.1%. Males posses 5 pairs of autosomes and one X chromosome, while hermaphrodites posses two X chromosomes in addition to the autosomal complement (the XX vs. XO system). Males are distinguished from hermaphrodites by their smaller size and by the fan-like copulatory bursa at the tip of the male tail. A male culture can by propagated by mating males with hermaphrodites. Half the progeny produced by such cross-fertilization are male. In practice, a culture of wide-type males is constantly maintained and these males are used for mating with mutant hermaphrodites. Such crosses produce heterozygous males that are then used to transfer the mutant marker to other hermaphrodites, so that genetic mapping and complementation tests are possible.<br> | Reproduction by self-fertilization, though convenient for mutant isolation and maintenance, does not provide a means to recombine independently isolated mutations. Genetic analysis therefore depends on the existence of males, which are produced spontaneously in hermaphrodite populations by meiotic non-disjunction at a frequency of 0.1%. Males posses 5 pairs of autosomes and one X chromosome, while hermaphrodites posses two X chromosomes in addition to the autosomal complement (the XX vs. XO system). Males are distinguished from hermaphrodites by their smaller size and by the fan-like copulatory bursa at the tip of the male tail. A male culture can by propagated by mating males with hermaphrodites. Half the progeny produced by such cross-fertilization are male. In practice, a culture of wide-type males is constantly maintained and these males are used for mating with mutant hermaphrodites. Such crosses produce heterozygous males that are then used to transfer the mutant marker to other hermaphrodites, so that genetic mapping and complementation tests are possible.<br> | ||
| Line 42: | Line 42: | ||
[[Image:Caenorhabditis male.jpg]]<br> | [[Image:Caenorhabditis male.jpg]]<br> | ||
<br> | <br> | ||
The majority of the mutants characterized | The majority of the mutants characterized to date are non-lethal and display a clearly visible phenotype. A large number of mutants are "uncoordinated" (now representing nearly 100 genes). Uncoordinated phenotypes range from small aberrations in movement to nearly complete paralysis. There are some great movies of hermaphrodite, male and mutant movement at [http://130.15.90.245/c__elegans_movies.htm Dr. Ian Chin-Sang's website]. | ||
Morphological mutants include dumpy, small, long, and blistered animals. Dumpy mutants are shorter than wild-type animals and correspond to twenty different genes dispersed over the six linkage markers and are useful for mapping most other classes of mutants, since the double mutants are easily distinguished. Both morphological and uncoordinated mutants can be used for mapping many types of developmental mutants. | |||
An increasing variety of mutations are now being studied including those affecting drug-resistance, chemotaxis, thermotaxis, male sexual behavior, catabolic pathways, dopamine biosynthesis, muscle assembly, sex determination, development of the ventral nerve cord, and temperature-sensitive lethal mutants affecting embryogenesis and gonadogenesis. A series of translocations and duplications has been characterized as a first step in assembling a collection of "balancers" for recessive lethal mutations. | An increasing variety of mutations are now being studied including those affecting drug-resistance, chemotaxis, thermotaxis, male sexual behavior, catabolic pathways, dopamine biosynthesis, muscle assembly, sex determination, development of the ventral nerve cord, and temperature-sensitive lethal mutants affecting embryogenesis and gonadogenesis. A series of translocations and duplications has been characterized as a first step in assembling a collection of "balancers" for recessive lethal mutations. | ||
Maintaining genetic stocks is less difficult with ''C. elegans'' than with some other organisms used in developmental or behavioral studies, such as ''Drosophila'' or the mouse. Stocks of ''C. elegans'' remain viable when frozen and stored in liquid nitrogen. | Maintaining genetic stocks of mutants and wild type strains is less difficult with ''C. elegans'' than with some other organisms used in developmental or behavioral studies, such as ''Drosophila'' or the mouse. Stocks of ''C. elegans'' remain viable when frozen and stored in liquid nitrogen. | ||
Consult [http://elegans.swmed.edu/ Leon Avery's ''C. elegans'' server] and [http://www.wormbase.org/ Wormbase] for more information about the molecular genetics of the worm. | |||
<br><br> | <br><br> | ||
Revision as of 17:09, 31 July 2009
Introduction to Caenorhabditis elegans
Although many of the aspects of gene function that have been studied extensively in prokaryotes pertain also to eukaryotes, prokaryotic cells cannot be used to investigate some questions specific to the biology of more derived organisms: chromosome structure and the development of a complex anatomy are key examples. The small, free-living soil nematode Caenorhabditis elegans is a convenient model system in which to approach basic questions concerning the genetic control of development and behavior in multicellular organisms. This animal's short life cycle is advantageous for genetic study, and, at the same time, it is small enough to allow complete anatomical studies at the ultrastructural level. C. elegans is easily and inexpensively grown in the laboratory in liquid medium or on the surface of agar-filled petri dishes, where it feeds on bacteria such as the genetically malleable species Escherichia coli (E. coli). Adult C. elegans worms are about 1 millimeter in length, with a tubular body consisting of a hypodermal wall and an underlying musculature that encloses the digestive and reproductive organs. Although C. elegans exibits most major types of differentiated tissues (nerve, muscle, hypodermis, intestine, and gonad), each adult worm contains only 959 somatic cells.
For further information, two major worm/web meccas are the web sites Leon Avery's C. elegans server and Wormbase. They can probably satisfy all your worm gene needs.
Nematode Anatomy
The hypodermal body wall of C. elegans is covered by an external cuticle composed primarily of modified forms of collagen. The body cavity (or pseudocoelom) is maintained at a high hydrostatic pressure relative to the outside, and this pressure on the elastic cuticle gives the animal rigidity and structural integrity. The hypodermis consists of four longitudinal ridges (dorsal, ventral, left and right lateral) joined circumferentially by thin sheets of cytoplasm which separate the muscle cells from the cuticle. The hypodermal cells secrete cuticular components and display a periodic activity associated with molting. The body muscles are organized into four longitudinal rows of 24 cells each, located between the hypodermal ridges. When the animal moves, the two subventral muscle strips are coordinated together, as are the two subdorsal strips, producing a sinusoidal wave motion in the dorsal-ventral plane. The alimentary canal includes a muscular pharynx, which takes up food (bacteria), grinds it, and pumps it into the intestine. The intestine consists of 30 to 34 cells; it is lined with microvilli leading to an anal sphincter valve that is operated by a pair of muscles.
The nervous system of C. elegans consists of approximately 300 neurons, including a circumpharyngeal nerve ring, dorsal and ventral nerve cords, and a variety of sensory receptors and ganglia. About 70% of the neurons are located in the head. Sense organs (sensilla) are arranged in two concentric rings around the mouth and include both chemoreceptors and mechanoreceptors. C. elegans exhibits chemotaxis to a number of compounds. It also responds to a tap on the head by moving backwards, and then by turning to move forward.
Nematode Lifecycle
Embryonic Development
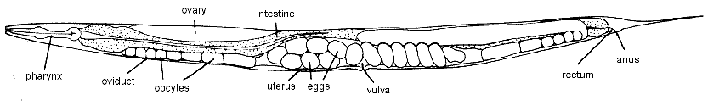
The development of C. elegans is known in great detail because this tiny organism (1 mm in length) is transparent and the developmental pattern of all 959 of its somatic cells has been traced. C. elegans generally reproduces as a self-fertilizing hermaphrodite, with each animal producing BOTH sperm and eggs. Two reflexed gonadal arms (each of which contains an ovary, oviduct, spermatheca, and uterus) terminate at the vulva, located midway along the ventral side. The mature hermaphrodite is effectively a female because the gonad has earlier made and stored sperm before turning to the production of oocytes. Eggs (developing embryos) are fertilized by endogenous sperm and then undergo some development inside the parental hermaphrodite. Embryos are arranged along the uterus with the most developed ones found nearest the vulva. Fertilized eggs are covered with a chitinous shell and, when mature enough, are deposited on the agar surface. Embryogenesis occurs in about 12 hours.
Post-Embryonic Development
A newly hatched animal resembles an adult in general proportions and body movement, but is only about one-sixth the length of an adult. During growth, developing larvae undergo four molts in which the old cuticle is shed and replaced by a new underlying cuticle. The inter-molt stages are designated L1 through L4, followed by adulthood. L3 and L4 hermaphrodites can be distinguished from adults via the presence of half-moon shaped structure at the location of the vulva. Worms stop pharyngeal pumping and become lethargic while molting. During growth at 20°C, molting occurs at approximately 14, 22, 30, and 40 hours after hatching, with egg laying commencing at about 50 hours. During larval development, the number of non-gonadal cells increases from about 550 in the newly hatched L1 to about 810 in the mature hermaphrodite and to about 970 in the mature male. At hatching the L1 has only 4 gonadal cells. These cells proliferate to form the adult reproductive system of approximately 2500 nuclei. It is important that you develop some ability to stage animals (that is; recognize the various developmental stages). This is most easily accomplished by simply viewing a mixed population such that you can directly compare the sizes of the various stages.
In summary, development from external egg deposit to the adult stage occurs in 2.5 days, and the worm's life span is 2-3 weeks. The entire life cycle takes about 3 1/2 days at 20°C with each adult hermaphrodite producing 250-350 progeny.
Nematode Genetics
The molecular genetics of C. elegans is well developed. This nematode holds the distinction of being the first metazoan to have its genome sequenced. In addition, most of the tricks of the molecular geneticist have been employed in C. elegans, including making transgenics (including knockouts) and the technique of RNA interference: RNAi. The small size of C. elegans (1mm), its short generation time (3 1/2 days at 20°C), as well as the large number of progeny produced per animal (250-350) are also important factors in genetic analysis. Since genetic experiments frequently require the detection of rare events, it is an advantage to grow a large number of individuals in a small space. A petri plate seeded with a single hermaphrodite will contain nearly 105 individuals after one week. Confining the animals to the 2-dimensional agar surface permits the observation of rare individuals in large populations with the aid of a dissecting microscope.
Self-fertilization drives populations to homozygosity so that it is easy to isolate isogenic (genetically identical) clones of animals. This greatly facilitates the detection of mutants in a diploid organism. Since chemically induced mutations first appear in the heterozygous condition, recessive mutations cannot be recognized in the F1 progeny of mutagenized animals. In C. elegans, heterozygous hermaphrodites automatically segregate homozygous mutants as one fourth of their progeny, compared to “male/female” organisms where segregation of homozygous mutants requires manual cloning (brother-sister matings) and where homozygotes only appear in the third generation after mutagenesis. Additionally, C. elegans hermaphrodites may be grown and screened for mutants together, since there is no danger of losing the homozygous form of a mutant by cross-fertilization. It is also advantageous that self-fertilization does not require copulation, allowing severely uncoordinated or deformed mutants to be propagated as homozygotes. Thus, many mutations that would be lethal in Drosophila or in the mouse are viable in C. elegans. This not only simplifies maintenance of genetic stocks, but it makes possible the growth of large populations of such mutants for biochemical analysis.
Reproduction by self-fertilization, though convenient for mutant isolation and maintenance, does not provide a means to recombine independently isolated mutations. Genetic analysis therefore depends on the existence of males, which are produced spontaneously in hermaphrodite populations by meiotic non-disjunction at a frequency of 0.1%. Males posses 5 pairs of autosomes and one X chromosome, while hermaphrodites posses two X chromosomes in addition to the autosomal complement (the XX vs. XO system). Males are distinguished from hermaphrodites by their smaller size and by the fan-like copulatory bursa at the tip of the male tail. A male culture can by propagated by mating males with hermaphrodites. Half the progeny produced by such cross-fertilization are male. In practice, a culture of wide-type males is constantly maintained and these males are used for mating with mutant hermaphrodites. Such crosses produce heterozygous males that are then used to transfer the mutant marker to other hermaphrodites, so that genetic mapping and complementation tests are possible.
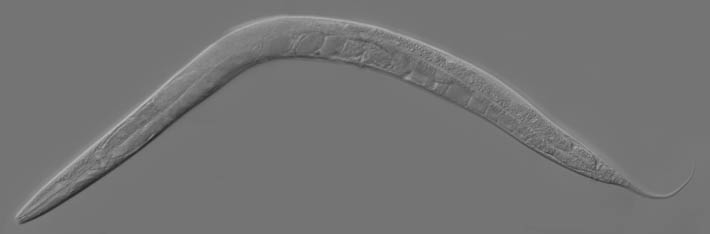
Adult C. elegans hermaphrodite
Adult C. elegans male
The majority of the mutants characterized to date are non-lethal and display a clearly visible phenotype. A large number of mutants are "uncoordinated" (now representing nearly 100 genes). Uncoordinated phenotypes range from small aberrations in movement to nearly complete paralysis. There are some great movies of hermaphrodite, male and mutant movement at Dr. Ian Chin-Sang's website.
Morphological mutants include dumpy, small, long, and blistered animals. Dumpy mutants are shorter than wild-type animals and correspond to twenty different genes dispersed over the six linkage markers and are useful for mapping most other classes of mutants, since the double mutants are easily distinguished. Both morphological and uncoordinated mutants can be used for mapping many types of developmental mutants.
An increasing variety of mutations are now being studied including those affecting drug-resistance, chemotaxis, thermotaxis, male sexual behavior, catabolic pathways, dopamine biosynthesis, muscle assembly, sex determination, development of the ventral nerve cord, and temperature-sensitive lethal mutants affecting embryogenesis and gonadogenesis. A series of translocations and duplications has been characterized as a first step in assembling a collection of "balancers" for recessive lethal mutations.
Maintaining genetic stocks of mutants and wild type strains is less difficult with C. elegans than with some other organisms used in developmental or behavioral studies, such as Drosophila or the mouse. Stocks of C. elegans remain viable when frozen and stored in liquid nitrogen.
Consult Leon Avery's C. elegans server and Wormbase for more information about the molecular genetics of the worm.
Tools and Techniques
Lab 1: Welcome to C. elegans
Lab 2: Mutant Hunt!
Lab 3: Linkage Test and Backcross
Lab 4: Linkage Test, Backcross and Mapping
Lab 5: Mapping Part 2
Lab 6: Score!