Talk:DNA ligation: Difference between revisions
No edit summary |
|||
| Line 31: | Line 31: | ||
#Crowe-NAR-1991 pmid=2011503 | #Crowe-NAR-1991 pmid=2011503 | ||
</biblio> | </biblio> | ||
==Ligation troubbles== | |||
[[Image:BHOG080507_gel1.jpg|thumb|500px|1% agarose gel, 0.5 mg/mL EtBr<br>0.5x TBE 0.5hrs@130V]] | |||
*'''[[User:Bjorn Hogberg|Bjorn Hogberg]] 14:13, 7 May 2008 (EDT)''':Hi, I'm having some real difficulties with my ligations (and/or transformations). The goal is to insert a 1.4kB PCR amplified insert into a 7.2kB M13mp18 vector cut with BamHI and HindIII. I have tried a lot of different strategies including: gel purification of insert vs. PEG purification of insert, room temp ligation vs. 16°C and 4°C ligations, ligation overnight vs. just an hour, Antrarctica Phosphatase treatment of vector vs. no treatment and a bunch of other stuff like varying ratios vector:insert. This gel is results from a 4°C overnight ligation (T4 DNA ligase from NEB) on Ant.Phosph. treated vector, I use 50ng of vector in a 20µl volume. It seems like the insert is ligating to itself a lot but never to the vector. Any suggestions, I'm desperate. | |||
I can also say that I have tried transforming on several occations. Once I thought it was alright because I got very few plaques on a negative control. And a lot of plaques on a 1:10 ligation plate, but alas, no insert was found after screening. | |||
Revision as of 18:13, 7 May 2008
General ligation protocols are fine for protruding sticky end ligations but often fail with blunt ends or recessed sticky ends. For blunt ends, higher enzyme concentrations are recommended along with considerably lower total DNA concentrations (cf. Bercovich et al., BioTechniques 12(2), 190-193 (1992)). If there is someone around with a good working protocol for ligation of blunt and of protruding ends, it would be much appreciated. {MDolinar, July 25, 2007}
Insert to Vector ratios
Hi, new to this wiki but not to wiki's. I notice that a formula was used for a 6:1 insert:vector ratio at the top of the page but later it talks about the appropraite range is 1:1 to 5:1, this seems like a contradiction. Under what conditions does one use the 6:1 ratio. This page needs at least some discussion of how to ligate vectors to inserts where both are reasonably long. Phil 18:22, 6 September 2007 (EDT).
- Reshma 19:50, 6 September 2007 (EDT): Good catch. I think this may reflect the fact that depending on exactly what you are ligating, the optimal insert:vector ratio can differ so different people advocate different ratio's. Hence the note in bold regarding the insert:vector ratio. If you have suggestions on ligating long inserts, please feel free to add it to the Notes section.
- Not suggestions but questions. I apparently have a setup that is hard to insert. V6.3kb:I2.2kb and have only the slightest evidence that V:I has formed, let alone circularized. I am noticing in the literature that optimal ligations are between 4 and 16'C (O/N) and that ligation ratios are between 1:1 for 'cip' treated vectors to 5:1 for blunt end ligations. Lots of contradictions, also people recommend vector concentrations of 10 fMole and insert of 30 fmole, but then say don't add to much DNA, no more than 20 ng per. Some people recommend preheating the vector and insert after mixing 37'C, 50'C, 65'C (drive iff residual ethanol or unstick the ends). For the first time user it is helpful to know what is most important (fmoles/ul or ngs/ul), what to do and when to do it.
- Reshma 14:21, 7 September 2007 (EDT): Ahh ok. How is your insert generated? By PCR followed by digestion or by digesting it from another vector? If it is digested from another vector, does the insert vector and the ligation vector have the same resistance marker? Have you've tried this cloning once already and it failed? If so, can you describe your conditions?
- Phil D 12:25, 9 September 2007 (CDT)There have been several things I have tried to clone, the vector can be cut and recloses with efficiency, which can be verified by cip treating it. The ligation of smaller products without transformation was successful, amplified by PCR. Several vector/insert products have been attempted, no success.
- Reshma 14:21, 7 September 2007 (EDT): Ahh ok. How is your insert generated? By PCR followed by digestion or by digesting it from another vector? If it is digested from another vector, does the insert vector and the ligation vector have the same resistance marker? Have you've tried this cloning once already and it failed? If so, can you describe your conditions?
- Not suggestions but questions. I apparently have a setup that is hard to insert. V6.3kb:I2.2kb and have only the slightest evidence that V:I has formed, let alone circularized. I am noticing in the literature that optimal ligations are between 4 and 16'C (O/N) and that ligation ratios are between 1:1 for 'cip' treated vectors to 5:1 for blunt end ligations. Lots of contradictions, also people recommend vector concentrations of 10 fMole and insert of 30 fmole, but then say don't add to much DNA, no more than 20 ng per. Some people recommend preheating the vector and insert after mixing 37'C, 50'C, 65'C (drive iff residual ethanol or unstick the ends). For the first time user it is helpful to know what is most important (fmoles/ul or ngs/ul), what to do and when to do it.
- So I have gone back to basic principles. The vector was EcoR1 digested and Cipped, heat killed the cip w/EDTA then added more magnesium and BamH1 digested, this blocks the 5' end of the linearized vector from closing or duplexing. The PCR product was cut with Bgl II (exension of 8 nt) but not EcoR1. In the latest attempt at ligation I am following a protocol 0.2 units of Enzyme 10 fM of vector, 30 fm Insert in 30 ul overnight at 14'C. This latest ligation was semi-successful. Majority of insert was in dimers and some vector-insert was barely (required trans illuminator box to see) visible, no visible vector-vector dimers.
- Since lower temperature works better I will repeat, at 10'C for 24 hours and see if I can improve amount of product. It may be true that more insert forces more vector-insert product, this is the first time I have seen a ratio of 6:1 proposed. Has anyone had success using less PEG, might this improve the reactivity of vector ends?
- Phil D. I have finished two ligations with 6:1 vector ratio, 24 hours at 10'C and all (>90%) of the insert was converted insert-insert. No evidence based on the gel that insert vector formed. So the ligation is apparently working
with good efficiency, simply stated the vector and insert are not ligating. We are restructuring the vector site, removing the BamH1 site and replacing it with a Bgl II site.
- Reshma 11:28, 11 September 2007 (EDT): Hmm. I'm afraid I do my clonings pretty differently from what you've outlined so it is hard for me to debug. It sounds as if it is not necessarily your ligation that is failing. It could be a combination of low efficiency cutting of your insert with low efficiency ligation equals very little product that is correctly ligated. Some ideas ...
- Phil D The efficiency problem is in the vector, it appears.
- Regarding PEG, NEB says that overnight ligations can inhibit transformation efficiency because of the PEG ... so can heat killing ligation reactions (heat treated PEG inhibits transformation efficiency). So it sounds like PEG impacts transformation but not necessarily ligation efficiency.
- One place where I have had problems in the past is getting efficient digestion of PCR products. Supposedly the theory is that polymerase can hang around attached to the DNA and prevent ligation (or fill in digested ends). My solution is to do a TOPO cloning of the PCR product, screen by colony PCR and subsequent sequencing of miniprepped DNA. Then digest the miniprepped DNA for cloning into the vector of my choice.
- Phil D. All inserts and vectors are cleaned up prior to RD. typically gel purification and DNA purification (Gene clean III). As I determined the insert-insert reaction works according to the book, at 10'C (which is optimal for those sticky and 24 hours resulted in near complete ligation.
It adds an extra cloning step but its worked for me where direct cutting of a PCR product and cloning failed. Alternatively, you could treat the PCR with proteinase K ... see reference below. I have not tried this approach but others in my lab have. However, based on the fact that your ligation of smaller products seems to be working and your latest ligation with BglII cut PCR product produced insert dimers, perhaps this is not the problem. (Although perhaps the EcoRI digest of the PCR product is less efficient or getting both BglII and EcoRI to cut your PCR product is less efficient?)
- The volume of the restriction digest can sometimes impact digest efficiency (according to my advisor ... he heard this from NEB). I do my restriction digests in a 50μL volume (small volumes ... say 20μL can be problematic apparently).
- I prefer using antarctic phosphatase rather than CIP. The antarctic phosphatase can be killed with heat as opposed to the EDTA you're using for CIP. Could the added EDTA be impacting the ligation?
- Phil D. Vector is purified after BamH1 digestion. Now that I have a ligation condition I know that works I am going to try it with vector only BamH1 cut, if that does not work then the MCS in the vector is the apparent problem. So . . . . If I can just get to the point of getting one closure I can re-PCR the vector-insert and cut with Eco-R1.
- I tend to do my ligations with 50ng vector and ~3-fold molar excess of insert. I am almost always doing 3-way ligations which is why I use so much DNA. I think you should be able to get away with less but these amounts work reliably for me.
I am not sure that any of these ideas are very helpful to you given the controls that you've done. If I come up with any more insights, I'll let you know.
- Crowe JS, Cooper HJ, Smith MA, Sims MJ, Parker D, and Gewert D. Improved cloning efficiency of polymerase chain reaction (PCR) products after proteinase K digestion. Nucleic Acids Res. 1991 Jan 11;19(1):184. DOI:10.1093/nar/19.1.184 |
Ligation troubbles
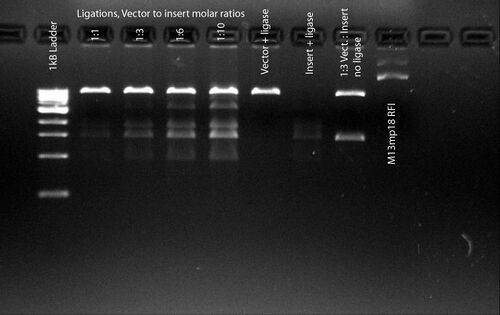
0.5x TBE 0.5hrs@130V
- Bjorn Hogberg 14:13, 7 May 2008 (EDT):Hi, I'm having some real difficulties with my ligations (and/or transformations). The goal is to insert a 1.4kB PCR amplified insert into a 7.2kB M13mp18 vector cut with BamHI and HindIII. I have tried a lot of different strategies including: gel purification of insert vs. PEG purification of insert, room temp ligation vs. 16°C and 4°C ligations, ligation overnight vs. just an hour, Antrarctica Phosphatase treatment of vector vs. no treatment and a bunch of other stuff like varying ratios vector:insert. This gel is results from a 4°C overnight ligation (T4 DNA ligase from NEB) on Ant.Phosph. treated vector, I use 50ng of vector in a 20µl volume. It seems like the insert is ligating to itself a lot but never to the vector. Any suggestions, I'm desperate.
I can also say that I have tried transforming on several occations. Once I thought it was alright because I got very few plaques on a negative control. And a lot of plaques on a 1:10 ligation plate, but alas, no insert was found after screening.