Sauer:bis-Tris SDS-PAGE, the very best: Difference between revisions
| Line 136: | Line 136: | ||
===Stacking:=== | ===Stacking:=== | ||
1X bis-Tris gel buffer, acrylamide solution to 5 %, water for remainder. This mixture can be stored alongside the resolving mix and APS as a "kit". | 1X bis-Tris gel buffer, acrylamide solution to 5-6 %, water for remainder. This mixture can be stored refrigerated alongside the resolving mix and APS as a "kit". | ||
Optional: Add Bromophenol Blue to make the stacking gel blue. It really helps when loading samples and doesn't affect the performance. You can fuss over the concentration, or just add it from a stock such that the gel in the casting has a decent blue color. | Optional: Add Bromophenol Blue to make the stacking gel blue. It really helps when loading samples and doesn't affect the performance. You can fuss over the concentration, or just add it from a stock such that the gel in the casting has a decent blue color. Having the stacking blue also helps to differentiate the stocks in your kit. | ||
I aliquot 2.5 mLs stacking per gel, which allows excess. | I aliquot 2.5 mLs stacking per gel, which allows excess. | ||
| Line 148: | Line 148: | ||
Add 10 μL TEMED per gel, mix, pour to fill the casting until slightly overflowing, insert the comb and leave the excess. Don't jam the comb all of the way in. The Bio-rad combs have small tabs that are intended as gap spacers. | Add 10 μL TEMED per gel, mix, pour to fill the casting until slightly overflowing, insert the comb and leave the excess. Don't jam the comb all of the way in. The Bio-rad combs have small tabs that are intended as gap spacers. | ||
Let polymerize. You'll notice a change in refraction as the wells polymerize up between the teeth of the comb. The polymerization typically stops before the surface and will not proceed to directly contact the comb (leaving rounded edges). Older protocols ask you to degas the solutions to promote polymerization, we found that this allows better polymerization, but accelerates the setting time to variable degrees, making it difficult to predict gel behavior. This is one reason to leave the excess solution on top. | Let the stack polymerize. You'll notice a change in refraction as the wells polymerize up between the teeth of the comb. The polymerization typically stops before the surface and will not proceed to directly contact the comb (leaving rounded edges). Older protocols ask you to degas the solutions to promote polymerization, we found that this allows better polymerization, but accelerates the setting time to variable degrees, making it difficult to predict gel behavior. This is one reason to leave the excess solution on top. | ||
Rinse with water to remove unpolymerized acrylamide. Remove comb. Be careful here, go slow. Creating too much suction or pushing the comb back in will cause the well spacers to unseat and bend. If this happens, you can carefully adjust their position with a gel loading tip once the system is set up. However, samples can then leak between lanes, so don't use it for a Western. Once assembled, carefully rinse unpolymerized acrylamide from the wells with a needle and syringe. We cut the tip off of the needle with scissors and reuse the same one over and over. | Rinse with water to remove unpolymerized acrylamide. Remove comb. Be careful here, go slow. Creating too much suction or pushing the comb back in will cause the well spacers to unseat and bend. If this happens, you can carefully adjust their position with a gel loading tip once the system is set up. However, samples can then leak between lanes, so don't use it for a Western. Once assembled, carefully rinse unpolymerized acrylamide from the wells with a needle and syringe. We cut the tip off of the needle with scissors and reuse the same one over and over. | ||
Revision as of 15:58, 2 March 2022
Submitted by Sean Moore
Based on work done by Tim Updyke and Sheldon Engelhorn for the Invitrogen Corporation (they bought Novex who developed it) and detailed in U.S. Patent 6,162,338. These gels are similar to those sold by Invitrogen as NuPAGE MES-Tris gels.
Background
The pH of the separating gel in “standard” SDS-PAGE (a.k.a. Laemmli buffer system) is roughly 8-9 which is conducive to the deamination and alkylation of proteins, as well as reoxidation of reduced cysteines during electrophoresis. What this means is that your protein will form disulfide crosslinks during the stacking event because the protein migrates into the gel away from the reducing reagent in the sample buffer, and gets focused to a high concentration. Acrylamide gels cast in alkaline buffers are also unstable during long term storage, breaking down to acrylic acid after 1 to 2 months resulting in loss of pore size, poor resolution, and modified proteins.
In this protocol, in-gel cysteine reoxidation is suppressed by casting and running under slightly acidic (~pH 6.5) conditions favoring cysteine protonation. Additionally, a reducing agent, sodium bisulfite, is included in the running buffer and will migrate into the gel and maintain a reducing environment. Another feature of this gel system is that the lower MW proteins near the buffer front do not accelerate towards the end of the run to the same degree as in Laemmli buffers. The result is higher resolution and a band distribution not unlike a gradient gel.
The Stacking and Resolving layers of the gel use the same buffer. This allows gels to be cast and stored for a long time (diffusion doesn't ruin the stacking chemistry). Also, the same tank running buffer is used at both the cathode and anode.
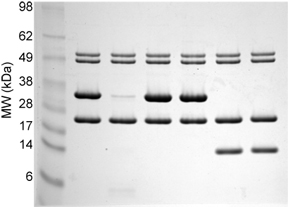
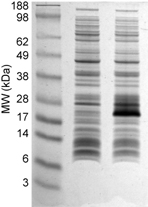
Sample bis-Tris MES-Tris Gels The first gel is of purified proteins. The second gel is of total E. coli lysate with and without an induced protein. Note the broad separation range of the molecular weight markers.
Materials
You can purchase pre-mixed MES and MOPS buffers from Boston Bioproducts. In this unusual case, it is cheaper than buying the individual components and mixing them yourself.
acrylamide
Buy or prepare a 30% acrylamide/bis-acrylamide stock. The amounts of bis-acrylamide typically varies in the stock depending on the size of proteins intended to be resolved and experience. Having the bis-acrylamide crosslinker at a lower concentration (e.g., 2.6 % found in 37.5:1 ratio mixtures) will allow larger pores to form and resolve larger proteins better. 3.3 % cross-linker (found in 29:1 ratio stocks) will form smaller pores. In practice, it's easier to use a 37.5:1 stock and change the final concentration (from ~12.5-15%) than it is to maintain several stocks. Alternatively, you can have a separate stock of dissolved bis-acrylamide and spike it into the final gel mixture to vary the cross-linker level.
If you are making your own stocks, use appropriate care when handling these toxins. Also, most deionized water sources have a low pH (from dissolved gas). This is good for acrylamide stocks and helps to prolong their shelf life, so don't adjust the pH with base (it isn't buffered and you'll cook your acrylamide).
5X low-MW running buffer
Use for separating small proteins 2-75 kDa.
250 mM MES
250 mM Tris
5 mM EDTA
0.5% SDS
No need to pH.
Oddly, this reagent can usually be found commercially as a pre-mixed solution that is cheaper than making it from scratch (the MES is expensive).
5X high-MW running buffer [optional]
use for separating proteins >20 kDa.
250 mM MOPS
250 mM Tris
5 mM EDTA
0.5% SDS
No Need to pH.
Also available commercially.
200X running buffer reducing agent
1 M sodium bisulfite
add to 1X running buffer at 5 mM final concentration. If the leftover buffer sat for a few days, you can refresh it with more bisulfite before use.
This solution will contain meta-bisulfite. Depending on the vendor, your chemical powder may be labeled that way on the bottle to reflect its presence. It's is the right stuff.
3X gel buffer
1.0 M bis-Tris (pH 6.5-6.8 with HCl)
Note: bis-Tris is Bis(2-hydroxyethyl) aminotris (hydroxymethyl) methane (e.g. Sigma catalog# B 7535).
We have changed our recipe from the original to make it easier to make and use. Prepare 1.0 M bis-Tris as a "3X". Using thirds helps when making the gel solutions in graduated conical tubes (e.g., pouring 15 mLs "by eye" to prepare 45 mLs of gel). Having the tank buffer and this gel buffer at pH 6.5 provides a more acidic environment and also more chloride ions than a pH 6.8 mixture, so try to get closer to 6.5, but don't sweat it.
sample buffer
You can use any of the the common Laemmli-like buffers. Many people over-cook their proteins and cause brakdown, probably because Laemmli was solubilizing a protein pellet. A short heating of ~1 min at ~80-100 C is more than sufficient and only has to be done once to fully denature the proteins and coat them. Using a lower temp, like 40-60 C to thaw frozen aliquots helps to prevent ruining the samples.
Stock reagents
Make separate 200 mM Tris-Cl, pH 6.8
Make separate 50% glycerol
Make separate 10% SDS
Make separate 0.1% bromophenol blue (may look a bit orange because of low pH, that's OK)
Aliquot some 2-mercaptoethanol into a microfuge tube, keep on hand (100% is ~14.3 M).
Mix the ingredients to some preferred "X", 2-3X is common:
2X
100 mM Tris-Cl, pH 6.8
20% glycerol
2% SDS
0.01% bromophenol blue
Store this concentrate and add 2-mercaptoethanol to ~4-5% (~140 mM) to an aliquot within a few days of use. There is a lot of 2-ME in many recipes because it needs to be in substantial excess over the cysteines to maintain them in a reduced state (and also compensating for air/oxygen oxidation of the 2-ME over time). There is no way to know the reducing activity in older preprarations; adding fresh to 1% a few times during the use of the aliquot is a cheat, but works fine for most applications.
Commercial 2X - 4X versions are found with different formulations, but most have the low pH Tris ranging from ~32 to 70 mM in the 1X, which provides Cl to aid in the stacking event.
The Laemmli original 1X is (Laemmli, 1970):
62.5 mM Tris-Cl, pH 6.8
2% SDS
10% glycerol
0.0006% bromophenol blue
5% 2-mercaptoethanol
Casting and running gels
Resolving:
Make sure you have enough 1X MES or MOPS tank buffer ready with relatively fresh sodium bisulfite added. When mixing from a cold 20X stock, the SDS is almost always out of solution, so mix the stock container well to distribute the SDS before pouring into the cylinder. When you add the water, it will re-dissolve.
Mix: 1/3 vol. of 3X bis-Tris gel buffer, acrylamide to 8% (30:2.0) or 12-15% (27.5:1), and water to final volume. I make 3.75 mLs for each Bio-Rad Protean gel, and use 3.5 mLs per gel. Unlike Tris-glycine gels, you can pre-mix this and keep it in the refrigerator for weeks, but the resolving quality will slowly deteriorate.
Add 25 μL of 10% APS per gel, mix in. Many protocols discuss having freshly prepared ammonium per sulfate. We haven't seen need for this and typically only refresh it when preparing new gel solution stocks.
Make sure your casting system and combs are ready...
Add 10 μL TEMED per gel and mix quickly. You can reverse the order of addition of the APS and TEMED if it's easier for mixing.
Pour the gel to the appropriate level (using a 5 mL pipette works well, but a 1 mL pipetter may be more convenient), tip/tap the gel as you go to remove bubbles that get stuck, and cover with water from a squirt bottle to the top of the casting (being careful to get an even, undisturbed interface).
Let polymerize (will become evident as a new refractive layer forming right below the gel/water interface).
Drain the water, tip the gel past 90 degrees to collect remaining water to an upper edge of the casting, and wick the excess water off using a Kim-wipe. Don't worry about every small drop.
Stacking:
1X bis-Tris gel buffer, acrylamide solution to 5-6 %, water for remainder. This mixture can be stored refrigerated alongside the resolving mix and APS as a "kit".
Optional: Add Bromophenol Blue to make the stacking gel blue. It really helps when loading samples and doesn't affect the performance. You can fuss over the concentration, or just add it from a stock such that the gel in the casting has a decent blue color. Having the stacking blue also helps to differentiate the stocks in your kit.
I aliquot 2.5 mLs stacking per gel, which allows excess.
Don't add APS until you are ready to start the polymerization. With this gel chemistry, the APS will start to polymerize the stacking gel if your resolving gel sets for a while and will be all goopy and unusable.
Add 15-20 μL 10% APS per gel, mix.
Add 10 μL TEMED per gel, mix, pour to fill the casting until slightly overflowing, insert the comb and leave the excess. Don't jam the comb all of the way in. The Bio-rad combs have small tabs that are intended as gap spacers.
Let the stack polymerize. You'll notice a change in refraction as the wells polymerize up between the teeth of the comb. The polymerization typically stops before the surface and will not proceed to directly contact the comb (leaving rounded edges). Older protocols ask you to degas the solutions to promote polymerization, we found that this allows better polymerization, but accelerates the setting time to variable degrees, making it difficult to predict gel behavior. This is one reason to leave the excess solution on top.
Rinse with water to remove unpolymerized acrylamide. Remove comb. Be careful here, go slow. Creating too much suction or pushing the comb back in will cause the well spacers to unseat and bend. If this happens, you can carefully adjust their position with a gel loading tip once the system is set up. However, samples can then leak between lanes, so don't use it for a Western. Once assembled, carefully rinse unpolymerized acrylamide from the wells with a needle and syringe. We cut the tip off of the needle with scissors and reuse the same one over and over.
If your system allows for it, rinse the casting with water before assembling the system (to remove unpolymerized acrylamide), and then remove the comb while the gel is in the tank covered with running buffer. This allows for the tank buffer the rush into the wells and rinse them and also lessens the chance of getting bubbles stuck in them.
Running
Add MES-Tris or MOPS-Tris buffer (with bisulfite) to the gel system. In our system, we dump the buffer into the top tank and let it overflow into the bottom chamber. Also, the tank markings suggest a huge volume of buffer is needed in the bottom chamber, but our gels run fine if we just cover the bottom edge of the gel by a few millimeters (there is a pair of round knobs in the Bio-rad frame that hold the bottom of the gel, just fill until those just get covered).
Carefully load your samples into the wells, letting the sample evenly settle on the well bottom and slowly raising the delivery height as the well fills (keep the delivery just below the sample/buffer interface). This method prevents a stray bubble from flying through the entire sample at the end of loading because the loading tip will be at the top by then.
- Note that Commercial protein markers will run to different positions in this system and you should get familiar with them. Also, the pre-stained markers have altered chemistries that make them particularly sensitive to changes in gel conditions. You should run a calibration gel with an unstained and a pre-stained marker side by-side to recalibrate your colored bands in your gel system. Once you have rune an unstained marker next to a common protein sample (like a bacterial lysate), you can use the lysate as your ladder for routine gels.
Run at constant current, about 25-35 mA per mini gel. If you're running two or more gels in the same tank, the temperature can get quite high and crack the plates. Experience will let you choose an appropriate current for your system. Take note of the buffer level in the top chamber and make sure it is not leaking while the gel runs. If it is, try to run the gel long enough to ensure the proteins are all in the gel, then stop the power, carefully pour the top tank buffer into the lower tank to keep it, unseat and re-seat each gel against the gaskets, carefully re-fill the top tank from the collected buffer, reassemble the wires and lid, and continue running.
After starting the run, you can switch the display to reveal the applied voltage, it should climb as the gel runs through the stacking event (as more conductivity establishes), then it will pause and slowly decline. In a 13% acrylamide, MES gel, The bromophenol blue runs around 3-5 kDa, which will leave the lower ~1/4 of the gel unused for a typical lysate separation. You can let the proteins spread out more by taking note of the bromophenol exit time and running the gel longer. This gel type causes the faster running portion to slow down as the run progresses, which allows the proteins to spread out more. Think of the coils of a stretched spring collapsing. Therefore, the amount of time needed to run "protein X" twice as far is not twice the time.
Staining
We use Coomassie extensively. The "Coomassie brilliant blue, R-250" makes a deep purple color with high contrast. The "G-250" type makes a softer blue with less contrast. We prefer the R-250.
Solutions
Stain
0.2-0.3 % Coomassie R-250, 10 % methanol, 10 % acetic acid, one liter: Weigh about 2-3 grams of Coomassie and place into a glass bottle.
Measure 100 mLs of methanol and carefully pour into the bottle (can rinse the weight boat if desired). Mix the solution until all of the Coomassie dissolves. If you have a bottle with volume markings, estimating the 100 mLs is okay.
Add water to 900 mLs.
Carefully add glacial acetic acid to make the liter volume. The acid promoted Coomassie aggregation and sticking to other surfaces (like proteins!), so add it last to help the Coomassie dissolve well.
Destain
10% acetic acid (no alcohol needed).
Procedure
Stop the gel, remove the power leads.
Disassemble the system and rinse the casting with deionized water (can be slippery, be careful).
Pour water into a plastic tray (tip box lid), about half a centimeter deep.
Carefully separate the gel plates (better to have someone show you this). The gel will stick to one side or the other.
Invert the plate/gel over the water and "convince" the gel to fall into the dish. It helps sometimes to put the gel and plate into the water and let the solution help the gel release.
place the gel on a rocker for a 2-5 minutes to remove excess free proteins (they leach from the gel surface).
Drain away the water (without dropping your gel in the sink), and cover with ~0.5 cm of strain solution.
Place the gel in stain in the microwave and microwave on high until the solution just begins to boil (this step greatly accelerates the procedure and allows you to see you bands in a minute or so).
Remove from the microwave and gently shake or rock for a few minutes. Once you see the gel filled with Coomassie, it's done.
Drain the Coomassie away and cover with water, rock for about 5 minutes, drain.
Cover with Destain solution and a couple of Kim-wipes folded flat over the gel.
Microwave again on high unit l the solution begins to boil.
Remove and shake/rock for several minutes. The Coomassie that is not bound to protein finds a new home on the Kim-wipe.
You can change out the Kim-wipe if you have excessive stain left.
The gel should be clear with dark purple protein bands.
If there is a Coomassie film on the surface, first image your gel, then try wiping the film off with a clean Kim-wipe before getting another image. This step can cause your gel to split, so it's better to get the image first.