Prince:FASP
From OpenWetWare
Jump to navigationJump to search
FASP Protocol
- Template File provides easy calculation of volumes and masses for Solution preparation
Steps
Cell/Tissue Samples:
- Preparing cells/tissue lysate
- Prepare SDT lysis buffer making sure you have a 1:10 sample:lysis-buffer ratio
- Bring this amount of lysis buffer to a boil and drop in frozen sample pellet, this is to reduce the activity of phosphotases and kinases as much as possible
- Incubate the lysed cells for 10 min at 95°C
- Sonicate the lysed cells (Branson SONIFIER 250 for 30 s) (Amplitude 40%) until no longer viscous (DNA broken)
- Incubate the mixture for 10 min at 95°C
- Clarify the crude extract by centrifugation at 16,000 x g at room temperature for 10 min.
- Sample processing (for complex sample) times are for 30K filters, when using large filters multiply volume amounts by 50
- load up to 40 µl (30μL is best) of supernatant with 200µl of UA buffer in 30k filters (Amicon ultra/ Vivacon 500) and vortex it
- concentrate the solution for 15 min at 14000 × g
- Add 100µl of UA and centrifuge the filters for 15 min at 14000 × g (twice)
- Add 100µl of UA-IAA, mix it well and incubate at room temperature for 20 min in dark. Spin out the UA-IAA reagent at 14000 × g for 15 min.
- Add 100µl of UA centrifuge the filters for 15 min at 14000 × g (twice)
- Add 100µl of ABC and centrifuge the filters for 20 min at 14000 × g (twice)
- Add trypsin (proteomic grade) to the sample in the ratio (1:40)
- mix the required amount of trypsin in 50µl of ABC and add to the filter, vortex it well and pulse the filters to 2000 × g
- Incubate the samples for 4 h-18 h at 37 °C
- Transfer the filter units to new collection tubes and centrifuge at 14000 × g
- wash the filter with 40µl of ABC and centrifuge at 14000 × g
- Acidify the solution to 1% formic acid
Purified Protein Samples:
- Sample processing (for purified proteins) times are for 30K filters
- load ~20 µg (have done up to 100) of purified protein in a 1:4 sample:UA buffer ratio in 30k filters and centrifuge for 15 min at 14000 × g
- Add 100µl of UA-DTT and mix the solution thoroughly centrifuge for 10 min at 14000 × g
- Add 100µl of UA and centrifuge for 10 min at 14000 × g
- Add 100µl of UA-IAA, mix it well and incubate at room temperature for 20 min in dark. Spin out the UA-IAA reagent at 14000 × g for 15 min.
- Add 100µl of UA centrifuge the filters for 15 min at 14000 × g (twice)
- Add 100µl of ABC and centrifuge the filters for 15 min at 14000 × g (twice)
- Add trypsin, (0.8 µg proteomic grade) to the sample in the ratio (1:40)
- mix the required amount of trypsin in 40 µl of ABC and add to the filter, vortex it well and pulse the filters to 2000 × g
- microwave (inverter based) the sample at 20% power for 1 min and let it cool to room temperature repeat the microwave process again.
- Transfer the filter units to new collection tubes and centrifuge for 15 min at 14000 × g
- wash the filter with 40µl of ABC and centrifuge for 15 min at 14000 × g
- Acidify the solution to 1% formic acid
Buffer Definitions
- UA
- 8M Urea in 0.1M Tris-HCl @ pH 8.5
- UA/DTT
- 0.1M DTT in UA buffer
- UA/IAA
- 50mM IAA in UA buffer
- SDT Lysis Buffer
- 4% w/v SDS, 0.1M DTT in 0.1M Tris-HCl @ pH 7.6
- ABC
- 50mM Ammonium Bicarbonate (in MS grade water)
Ordering Information
- Trypsin
- Sequencing Grade Trypsin from Promega (C/N #V5113) or Trypsin Gold, Mass Spectrometry Grade from Promega (C/N #V5280)
- Filters
- Sartorius stedim Vivacon 500 Line Vivacon Product Information
- 100K
- 50K
- 30K
- 10K requires additional 30 minutes/spin
- 2K we haven't tried yet
- Sartorius stedim Vivacon 500 Line Vivacon Product Information
Notes
Measuring peptide concentration in eluant
- 1mg/ml solution has 1.1 au at 280nm
Concentration of the peptides can be estimated by UV spectrometer assuming that 0.1% solution of vertebrate proteins has at 280 nm an extinction of 1.1 absorbance units.
Reference
FASP paper (from the supplement of Nature Methods: 6(5) 2009)
Trypsin
Why you do not want to have any residual reducing agent (e.g. DTT) in your digestion buffer:
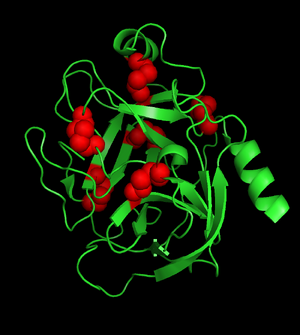
Porcine Trypsin [Green: Backbone, Red: Disulfide bonds]
In Gel FASP Protocol
Also, try our new and improved In-gel FASP method!