OSDDMalaria:GSK Arylpyrrole Series:desired compounds
The decisions about the compounds discussed below is being carried out at The Synaptic Leap
Finalised List
This finalised list of desired synthetic compounds is the result of the compounds consultation. The descriptors "sA" etc are temporary and will be replaced with appropriate designators when acquired in reality. We are also interested in the secondary and tertiary amide analogues of these compounds as well. for compound sH, other heterocycles would be of interest.
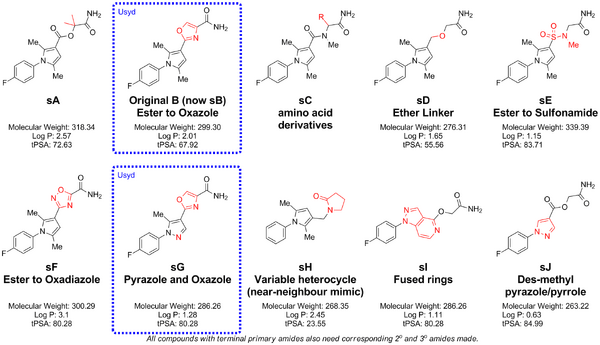
The .cdx file is available via a public dropbox
Synthetic SMILES/InChi
sA: FC1=CC=C(N2C(C)=CC(C(OC(C)(C)C(N)=O)=O)=C2C)C=C1 InChI=1S/C17H19FN2O3/c1-10-9-14(15(21)23-17(3,4)16(19)22)11(2)20(10)13-7-5-12(18)6-8-13/h5-9H,1-4H3,(H2,19,22)
sB: FC1=CC=C(N2C(C)=CC(C3=NC(C(N)=O)=CO3)=C2C)C=C1
InChI=1S/C16H14FN3O2/c1-9-7-13(16-19-14(8-22-16)15(18)21)10(2)20(9)12-5-3-11(17)4-6-12/h3-8H,1-2H3,(H2,18,21)
sC (alanine derivative shown): FC1=CC=C(N2C(C)=CC(C(N(C)C(C)C(N)=O)=O)=C2C)C=C1
InChI=1S/C17H20FN3O2/c1-10-9-15(17(23)20(4)12(3)16(19)22)11(2)21(10)14-7-5-13(18)6-8-14/h5-9,12H,1-4H3,(H2,19,22)
sD: FC1=CC=C(N2C(C)=CC(COCC(N)=O)=C2C)C=C1
InChI=1S/C15H17FN2O2/c1-10-7-12(8-20-9-15(17)19)11(2)18(10)14-5-3-13(16)4-6-14/h3-7H,8-9H2,1-2H3,(H2,17,19)
sE: FC1=CC=C(N2C(C)=CC(S(N(C)CC(N)=O)(=O)=O)=C2C)C=C1
InChI=1S/C15H18FN3O3S/c1-10-8-14(23(21,22)18(3)9-15(17)20)11(2)19(10)13-6-4-12(16)5-7-13/h4-8H,9H2,1-3H3,(H2,17,20)
sF: FC1=CC=C(N2C(C)=CC(C3=NOC(C(N)=O)=N3)=C2C)C=C1
InChI=1S/C15H13FN4O2/c1-8-7-12(14-18-15(13(17)21)22-19-14)9(2)20(8)11-5-3-10(16)4-6-11/h3-7H,1-2H3,(H2,17,21)
sG: FC1=CC=C(N2N=CC(C3=NC(C(N)=O)=CO3)=C2C)C=C1
InChI=1S/C14H11FN4O2/c1-8-11(14-18-12(7-21-14)13(16)20)6-17-19(8)10-4-2-9(15)3-5-10/h2-7H,1H3,(H2,16,20)
sH: O=C1CCCN1CC(C=C(N2C3=CC=CC=C3)C)=C2C
InChI=1S/C17H20N2O/c1-13-11-15(12-18-10-6-9-17(18)20)14(2)19(13)16-7-4-3-5-8-16/h3-5,7-8,11H,6,9-10,12H2,1-2H3
sI: FC1=CC=C(N2C(C=CN=C3OCC(N)=O)=C3C=N2)C=C1
InChI=1S/C14H11FN4O2/c15-9-1-3-10(4-2-9)19-12-5-6-17-14(11(12)7-18-19)21-8-13(16)20/h1-7H,8H2,(H2,16,20)
sJ: FC1=CC=C(N2N=CC(C(OCC(N)=O)=O)=C2)C=C1
InChI=1S/C12H10FN3O3/c13-9-1-3-10(4-2-9)16-6-8(5-15-16)12(18)19-7-11(14)17/h1-6H,7H2,(H2,14,17)
Commercially available analogues of desired compounds
This is a list of commercially available analogues of the above compounds. The cores of each of the different classes in the above list were searched by eMolecules.com and by supplier websites. Partial search carried out by via Molport.
The consultation did not largely change the desired commercial compounds. The thiazolidinone type compounds were discounted. The list of Smiles/InChi strings below reflects the desired commercial compounds
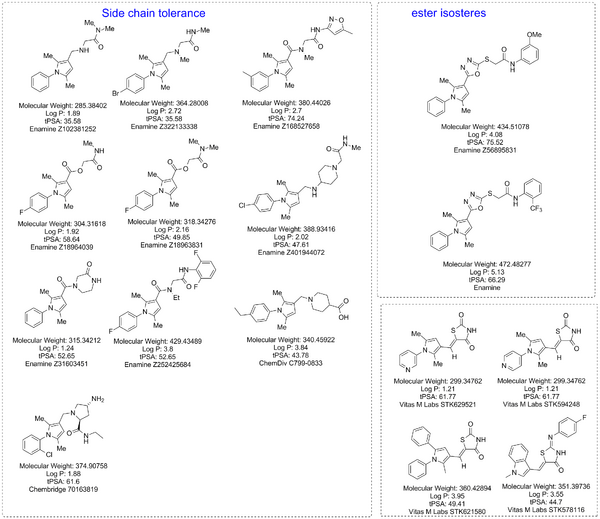
The .cdx file is available via a public dropbox
Commercial Smiles/InChi list
cA: O=C(N(C)C)CNCC1=C(C)N(C(C)=C1)C2=CC=CC=C2 InChI=1S/C17H23N3O/c1-13-10-15(11-18-12-17(21)19(3)4)14(2)20(13)16-8-6-5-7-9-16/h5-10,18H,11-12H2,1-4H3
cB: FC1=CC=C(N2C(C)=CC(C(OCC(N)=O)=O)=C2C)C=C1
InChI=1S/C15H15FN2O3/c1-9-7-13(15(20)21-8-14(17)19)10(2)18(9)12-5-3-11(16)4-6-12/h3-7H,8H2,1-2H3,(H2,17,19)
cC: O=C(N1CCNC(C1)=O)C2=C(C)N(C(C)=C2)C3=CC=CC=C3
InChI=1S/C17H19N3O2/c1-12-10-15(17(22)19-9-8-18-16(21)11-19)13(2)20(12)14-6-4-3-5-7-14/h3-7,10H,8-9,11H2,1-2H3,(H,18,21)
cD: O=C(NC)CN(C)CC1=C(C)N(C(C)=C1)C2=CC=C(Br)C=C2
InChI=1S/C17H22BrN3O/c1-12-9-14(10-20(4)11-17(22)19-3)13(2)21(12)16-7-5-15(18)6-8-16/h5-9H,10-11H2,1-4H3,(H,19,22)
cE: O=C(NC1=CC=CC(OC)=C1)CSC2=NN=C(O2)C3=C(C)N(C(C)=C3)C4=CC=CC=C4
InChI=1S/C23H22N4O3S/c1-15-12-20(16(2)27(15)18-9-5-4-6-10-18)22-25-26-23(30-22)31-14-21(28)24-17-8-7-11-19(13-17)29-3/h4-13H,14H2,1-3H3,(H,24,28)
cF: FC1=CC=C(N2C(C)=CC(C(N(CC)CC(NC3=C(F)C=CC=C3F)=O)=O)=C2C)C=C1
InChI=1S/C23H22F3N3O2/c1-4-28(13-21(30)27-22-19(25)6-5-7-20(22)26)23(31)18-12-14(2)29(15(18)3)17-10-8-16(24)9-11-17/h5-12H,4,13H2,1-3H3,(H,27,30)
cG: O=C(NC1=NOC(C)=C1)CN(C)C(C2=C(C)N(C(C)=C2)C3=CC(C)=CC=C3)=O
InChI=1S/C21H24N4O3/c1-13-7-6-8-17(9-13)25-14(2)10-18(16(25)4)21(27)24(5)12-20(26)22-19-11-15(3)28-23-19/h6-11H,12H2,1-5H3,(H,22,23,26)
cH: ClC1=CC=C(N2C(C)=CC(CNC3CCN(CC(NC)=O)CC3)=C2C)C=C1
InChI=1S/C21H29ClN4O/c1-15-12-17(16(2)26(15)20-6-4-18(22)5-7-20)13-24-19-8-10-25(11-9-19)14-21(27)23-3/h4-7,12,19,24H,8-11,13-14H2,1-3H3,(H,23,27)
cI: O=C(NCC)[C@H](C[C@@H](N)C1)N1CC2=C(C)N(C(C)=C2)C3=CC=CC=C3Cl
InChI=1S/C20H27ClN4O/c1-4-23-20(26)19-10-16(22)12-24(19)11-15-9-13(2)25(14(15)3)18-8-6-5-7-17(18)21/h5-9,16,19H,4,10-12,22H2,1-3H3,(H,23,26)/t16-,19+/m1/s1
cJ: CCC1=CC=C(N2C(C)=CC(CN3CCC(C(O)=O)CC3)=C2C)C=C1
InChI=1S/C21H28N2O2/c1-4-17-5-7-20(8-6-17)23-15(2)13-19(16(23)3)14-22-11-9-18(10-12-22)21(24)25/h5-8,13,18H,4,9-12,14H2,1-3H3,(H,24,25)
cK (additional): FC1=CC=C(N2C(C)=CC(C(OCC(N(C)C)=O)=O)=C2C)C=C1
InChI=1S/C17H19FN2O3/c1-11-9-15(17(22)23-10-16(21)19(3)4)12(2)20(11)14-7-5-13(18)6-8-14/h5-9H,10H2,1-4H3
Original List Desired Analogues
This is a list of compounds we are currently targeting or would like to have access to. If you happen to have some of these compounds, or their close analogues, in your labs or would like to make them then please get in touch. The compounds highlighted in red are current priority compounds, please get in touch on The Synaptic Leap with your views.
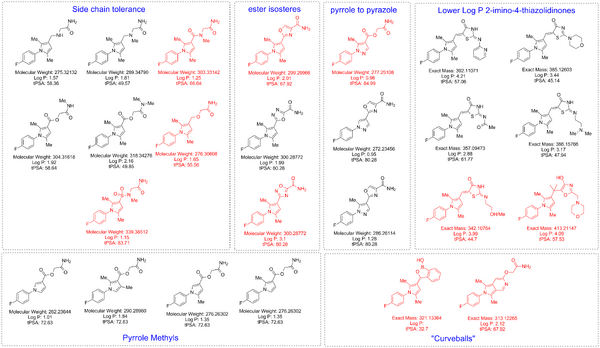
The .cdx file is available via a public dropbox
The near neighbour compounds could be available from the authors of this paper
SMARTS Filter
A SMARTS filter was carried out on the above compounds with the following summary (All fail on the Alarm NMR):
total: 24
passed: 6 (25.0%)
failed: 18 (75.0%)
failed glaxo_unsuitable_leads: 0 (0.0%)
failed glaxo_unsuitable_natprod: 0 (0.0%)
failed glaxo_reactive: 0 (0.0%)
failed ursu_reactive: 0 (0.0%)
failed lint_blake-v2: 1 (4.2%)
failed oprea_filters: 1 (4.2%)
failed MLSMR_excluded: 12 (50.0%)
failed MLSMR_allowed: 1 (4.2%)
failed toxic: 0 (0.0%)
failed pains: 15 (62.5%)
