McClean: Hemacytometer protocol for yeast
From OpenWetWare
Jump to navigationJump to search
Overview
This is a protocol for counting yeast cells using a hemacytometer. Use this protocol to measure the density of a culture in cells/ml. Can also be used with viability stains to test live vs dead cell number.
Materials
- Hemacytometer: A hemacytometer consists of a carefully calibrated glass slide that has a counting grid and a thick coverslip that is of a specific weight to cause cells to spread thinly along the surface. Together these are used to count the cell concentration of a sample in cells/ ml.
- Cell Culture
Protocol
Loading the Hemacytometer
- Clean the hemacytometer with 70% ethanol and make sure both the slide and coverslip are free from dust and debris.
- Place the coverslip over both the grid, leaving the indentation for loading uncovered.
- Vortex your cell solution so that cells are suspended. Also, your culture can't be too dense. If you pipette a saturated culture into the hemacytometer you will have too many cells to count. Experiment with a couple different dilutions if you need to.
- Pipette 10μL of sample into the hemacytometer by holding the pipette tip against the loading indentation.
Counting Cells
- Using the ?? microscope, and the ?? objective (someone fill this in once we figure it out) locate the squares and subsquares as shown in the figure.
- Count the center square which is divided into a 5X5 grid of squares (which are further divided into 16 subsquares). Count the four corner squares and the center square, 5 subsquares in total. DO NOT count cells that land on the right hand side line or the bottom line, but DO count cells that land on the top line or the left hand side line.
- You should count about 100 cells (so, you want your culture diluted to have about 20 cells per subsquare). If you need to count more subsquares to reach 100 cells do so. If your dilution is making counting difficult (too many or too few cells) go back and redo your dilution. This is faster and less annoying than counting 25 subsquares or having 200 cells per subsquare.
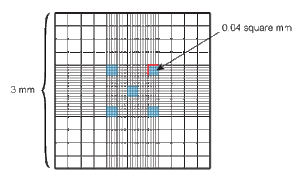
Calculations
- The middle large square measures 1mm X 1mm. The Reichert Bright-Line hemacytometer (is this our brand?) has a depth of 0.1mm. The large squares therefore have a volume of 0.1mm3.
- Please fill in the rest of the protocol. How do you go from the cell count in 5 different squares to average cells/ml? It is helpful to know that 1mm3=1μL
Stock Solutions
Stock Solution 1
- This is a very simple solution, so we only need a one line description of how to make it.
Stock Solution 2
This is a more involved solution, so we will describe how to make it in several steps:
- Step 1
- Step 2
- Step 3
Protocol
- Step 1
- Step 2
- Step 3
Notes
Please feel free to post comments, questions, or improvements to this protocol. Happy to have your input!
- List troubleshooting tips here.
- You can also link to FAQs/tips provided by other sources such as the manufacturer or other websites.
- Anecdotal observations that might be of use to others can also be posted here.
Please sign your name to your note by adding '''*~~~~''': to the beginning of your tip.
References
Gietz, R.D. and R.A. Woods. (2002) TRANSFORMATION OF YEAST BY THE Liac/SS CARRIER DNA/PEG METHOD. Methods in Enzymology 350: 87-96.
Contact
- Megan N McClean 14:01, 20 July 2011 (EDT)
or instead, discuss this protocol.