Mathies:Sieving Matrices
Overview
The sieving matrix is what makes electrophoresis of biomolecules work. In molecular biology, sieving matrices are usually agarose (for DNA) or cross-linked polyacrylamide (for proteins). Neither of these go into or out of a chip very well, so we use "replaceable" matrices like LPA, HEC, and PDMA. Basically, just solutions of linear polymers.
This section will introduce you to some theory of linear polymers and familiarize you with the methods for making effective sieving matrices from them (including radical-transfer polymerization). Start reading at HEC and continue on through LPA to PDMA.
There are plenty of other polymers that could, but won't be covered here. These include PEO, PVP, HPMC, and PVA. These are usually bought as dry powders and rehydrated in the exact same way as HEC.
HEC - the linear miracle polymer
HEC is a polymer of sugar molecules (D-glucose) that have nonionic hydroxyethyl groups added by ether linkages to the cellulose backbone. HEC is almost ubiquitous in its uses as an additive and thickener to cosmetics and foods. It’s also used as a general lubricant for applications from tear-deprived eyes to crude oil drilling rigs. You can find a good overview on celluloses here
Pro's and Con's
The advantages of using an HEC solution as a sieving matrix are:
• Non-Toxic
• Easily dissolves in water
• Low-viscosity
• Low-fluorescence background
The disadvantages of using an HEC solution as a sieving matrix are:
• So-so sieving performance (~5-bp resolution max)
• Contains particulates so filtration is necessary
• Not so anionic (unsuitable for IEF)
Making an HEC Solution
HEC solutions for CE are extremely easy to make. Typical percentages are 0.75% to 1.25% w/v.
To make a 100 mL stock solution of 0.75% HEC:
1) Prepare 100 mL of the buffer you wish to use (e.g. 1x TBE)
2) Weight out 0.75 g of HEC. Make a note of which type you use as they all perform differently. Charlie uses 250HR CS.
3) Pour the buffer into a 150 mL Wheaton (or similar) bottle
4) Add a magnetic stir bar and place on a stir plate at low speed
5) Slowly add the HEC powder. If you add it too quickly, it will clump at the surface and take days to dissolve.
6) Cap and stir overnight. Gentle heating will accelerate the dissolution.
7) Filter through a 5 um syringe filter for best results.
- HEC solutions can be stored for years without significant effect of performance.
- Do not add the HEC powder before the buffer solution. This will leave a difficult-to-dislodge HEC plug at the bottom of the bottle that the stir bar will only slightly graze.
Sieving Theory for HEC
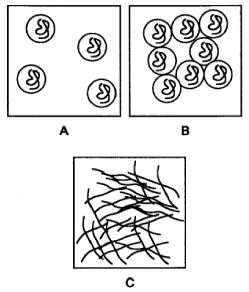
As we use it, HEC is a polydisperse linear polymer that is soluble in water. Like most soluble linear polymers, it adopts a random coil structure when in water. This structure is “free-draining”, or open enough to easily allow passage of water into and out of the polymer coil. At very low concentrations, individual polymer molecules will exist in solution independent of each other (Figure A). As the concentration is increased, these individual coils will start bumping into each other and “entangling” (Figure B). Keep increasing the concentration and eventually every polymer coil will always be bumping into a neighbor, forming a continuous network (Figure C). This is the entanglement threshold. Electrophoresis benefits from rigid, but permeable polymer networks for sieving. Because of this, electrophoresis is run above the entanglement threshold. Not by much, as viscosity really takes off after the entanglement threshold. An entangled linear polymer solution should have pores that are about as large as the free-draining paths through an individual HEC coil. Fortunately, celluloses are pretty rigid and these pores are concomitantly large – large enough for DNA to pass through, but small enough to slow everything down.
LPA Synthesis
Solutions of LPA are an excellent sieving matrix and are most commonly used where very-high resolution is required (e.g. DNA sequencing, STR typing). LPA can be easily synthesized by a radical addition polymerization of acrylamide monomer. The sieving performance of LPA is a function of the polymer length (generally, the longer the better) and the polymer length depends greatly on the polymerization conditions. Thus, it’s really easy to make bad LPA, but takes some skill and practice to make good LPA.
Reagents and Equipment Needed
• Acrylamide (monomer) – electrophoresis (or greater) purity
• MQ H2O
• TEMED, 20% v/v in MQ H2O
• Ammonium Persulfate (10% w/v solution made fresh or frozen)
• 20 mL scintillation vial
• Rubber septum
• 10 uL Hamilton syringes
• Argon Gas
LPA Synthesis Protocol
LPA is synthesized by mixing acrylamide monomer with buffer, sparging to remove O2, and finally adding the polymerization initiator and catalyst. LPA is pretty runny under 3% w/v and is nearly solid over 7.5% w/v. Typical concentrations are 3-5% w/v.
To make a 10 mL solution of 3.5% w/v LPA:
1) Measure 0.35 g of acrylamide, and add water/buffer to 10 mL in the scintillation vial. The acrylamide will dissolve very quickly. Be careful when doing all this, because the acrylamide monomer is a nasty neurotoxin.*
2) Cap the scintillation vial with the rubber septum and sparge gently with argon for 2-3 hours. It is absolutely critical that all oxygen is removed from the solution during sparging, so don’t use an old, beat up septum.
3) After 2-3 hours, pull the sparging needle out of the acrylamide solution so that the argon simply blows over the solution.
4) Using the Hamilton syringe, pierce the septum and inject 5 uL of the TEMED solution directly into the acrylamide solution.
5) Using the Hamilton syringe, again pierce the septum and inject 5 uL of the 10% APS solution directly into the acrylamide solution.
6) Immediately swirl the scintillation vial to thoroughly mix the APS and TEMED with the acrylamide solution. Don’t swirl so vigorously that you introduce gas to the solution, and make sure that the argon is flowing the entire time.
7) Leave with argon flowing for 30 min.
8) Remove sparging needle and vent needle, wrap the septum with Parafilm and leave overnight to finish polymerization.
- At this point, the acrylamide monomer solution can be filtered with a syringe filter, but this is generally not necessary.
Pro's and Con's
The advantages of using a LPA solution as a sieving matrix are:
• Very hydrophilic polymer
• Very high resolution separations
• Zero-fluorescence background
• Mostly particulate free
The disadvantages of using a LPA solution as a sieving matrix are:
• Acrylamide is a potent neurotoxin!
• Tricky to synthesize
• High viscosity
• Unstable over time (deamination makes it anionic)
Poly N,N-dimethylacrylamide (PDMA)
Solutions of PDMA are very good sieving matrices with low viscosities and dynamic-coating properties. PDMA (like LPA) can be easily synthesized by a radical addition polymerization of N,N-dimethylacrylamide (DMA) monomer. The synthesis, performance, and use of PDMA is almost identical to that of LPA with only a few exceptions. I’ve found 5% PDMA (with 1% IPA) to be a great replacement for HEC.
Reagents and Supplies Needed
• N,N-dimethylacrylamide (DMA monomer) – it’s a liquid
• MQ H2O
• TEMED
• Ammonium Persulfate (10% w/v solution made fresh or frozen)
• 20 mL scintillation vial
• Rubber septum
• 10 and 50/100 uL Hamilton syringes
• Argon Gas
• Isopropyl Alcohol (optional)
PDMA Synthesis
PDMA is synthesized by mixing DMA monomer with buffer, sparging to remove O2, and finally adding the polymerization initiator and catalyst. PDMA is thick at 5% and almost solid at 10%, but it exhibits more of a continuum of viscosities than LPA. Typical concentrations are 5-10% v/v. NB – this procedure is almost exactly the same as that for LPA; only the reagents differ.
To make a 10 mL solution of 5% v/v PDMA:
1) Add 500 uL DMA to 9.5 mL water/buffer in the scintillation vial. The acrylamide will dissolve very quickly.** Be careful when doing all this, because the DMA monomer is toxic and flammable.
2) Cap the scintillation vial with the rubber septum and sparge gently with argon for 2-3 hours. It is absolutely critical that all oxygen is removed from the solution during sparging, so don’t use an old, beat up septum.
3) After 2-3 hours, pull the sparging needle out of the acrylamide solution so that the argon simply blows over the solution.
4) Using the Hamilton syringe, pierce the septum and inject 5 uL of the TEMED solution directly into the acrylamide solution.
5) Using the Hamilton syringe, again pierce the septum and inject 25 uL of the 10% APS solution directly into the acrylamide solution.
6) Immediately swirl the scintillation vial to thoroughly mix the APS and TEMED with the acrylamide solution. Don’t swirl so vigorously that you introduce gas to the solution, and make sure that the argon is flowing the entire time.
7) Leave with argon flowing for 30 min.
8) Remove sparging needle and vent needle, wrap the septum with Parafilm and leave overnight to finish polymerization.
- IPA can be added before sparging to reduce the mean polymer chain size. IPA is an effective chain transfer agent – meaning that it can transfer a radical from a growing polymer chain to another chain or to a monomer. This reduces the viscosity further without seriously effecting sieving performance. I typically use 1% v/v IPA.
Pro's and Con's
The advantages of using a PDMA solution as a sieving matrix are:
• Low viscosity (vs HEC or LPA)
• Very high resolution separations
• Zero-fluorescence background
• Mostly particulate free
The disadvantages of using a LPA solution as a sieving matrix are:
• DMA is toxic and flammable!
• Tricky to synthesize
• High viscosity
• Unstable over time (but not as much as LPA)
What Makes PDMA So Special...
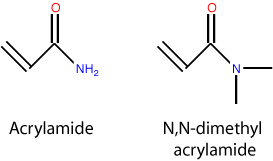
PMDA is structurally and chemically very similar to LPA but exhibits different properties like lower viscosity. Why? As shown in the figure, the DMA monomer has two methyl groups attached to the nitrogen, where the acrylamide monomer has only hydrogens. In the case of acrylamide, this amine group is excellent at hydrogen bonding and, along with the carboxyl, make it very hydrophilic. For DMA, the methyl groups substantially lower the polarity of the molecule, making DMA much less hydrophilic than acrylamide. This is not to say that DMA or PDMA are hydrophobic – they are not, and remain soluble to very high concentrations. When polymerized, the lower hydrophilicity of PDMA results in polymers that like to associate with themselves more than water (with respect to LPA). This raises the entanglement threshold and gives a lower viscosity. An excellent treatment of LPA, PDMA and some other variants is given by Heller (Electrophoresis, 1999).
Another interesting property of PMDA solutions is that they provide a “dynamic” coating that is similar in effect to the Hjerten coating. It’s not well understood what gives rise to the dynamic coating effect (at least I don’t understand it), but it works well enough that it is used by ABI in all of their capillary instruments. Some treatment with acid and base must be done to the capillaries or channels before hand, but my limited experiments with dynamic coating have all been failures. Hence, I use PDMA with Hjerten-coated channels and it works very well.
Contact
- Eric Chu 22:00, 22 July 2009 (PDT):
or instead, discuss this protocol.