Mathies:Calibration Curves
CALIBRATION CURVE (E. COLI CULTURE IN PLATES AND QUANTIFICATION)
Suggestion: Calibration Curves should be verified periodically using fresh plates in order to prevent mis-quantification
Reagents
Bacteria: E. coli strain K12 and O157
Plates: LB plates/blood agar plates, LB plates containing 100µg/ml Ampicillin, and LB plates containing 100µg/ml Spectinomycin (from Tekeno)
Protocol
1) Grow cells overnight in broth (See section “E. COLI CULTURE IN BROTH AND QUANTIFICATION” step (1) to Step (11))
2) Warm all plates at 37˚C for 30 minutes (the cover of the plates should face down side to prevent evaporation)
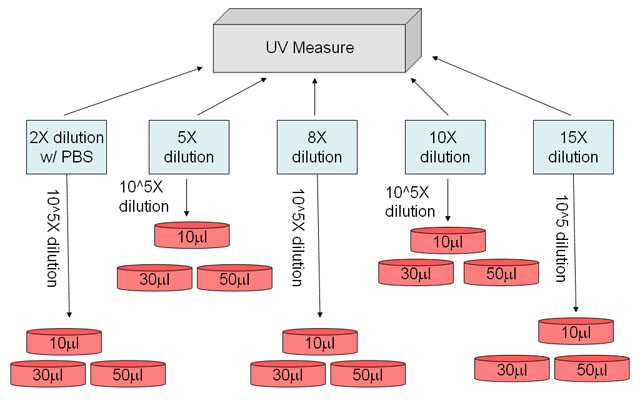
3) Make different cell dilutions with PBS (rule of thumb: the OD600 of the different dilutions should fall between 0.1~0.3 because this is our expected value from step (13) of section “E. COLI CULTURE IN BROTH AND QUANTIFICATION”. Please see suggested first dilution in the blue box of Figure 1)
4) Measure OD600 of the different dilutions by UV/VIS spectrophotometer, and make second dilution (suggested second dilution: 10^5X) of each first dilution before spreading the cell onto the selective plates
5) Spread different volumes of cell dilution onto selective plates using Spreader (Lewis 311 “Cell Growing Tool” drawer, from LSA P465)
6) Place all the plates in the incubator at 37˚C for overnight. The next day
7) Count and record CFU(cell forming unit) from each plates
8) Data extrapolation (Figure 2)
9) Most up-to-date calibration curves:
K12: Y=593110X-14377
O157: Y=673906.3X+160300.6
Where X=OD600 measurement of the 10X cell dilution, and Y=Number of CFU/µl of 10X cell dilution
Contact
- Eric Chu 22:25, 22 July 2009 (PDT):
or instead, discuss this protocol.