Lidstrom:Competent Cell Preparation
From OpenWetWare
Jump to navigationJump to search
Back to Protocols
Chemically Competent E. Coli
Notes:
- You need fresh cells.
- Often people inoculate a few mL of the culture for overnight growth, then use 200 uL to inoculate the ~50 mL of culture they will make competent.
- Some people in our lab believe you want to start with a fresh plate and don't even use one that is a few days old. Other people (Mila) are mostly concerned about the OD being right at the time of harvest.
- You need to flash freeze the cells at the end of the procedure. You can do this by pouring liquid nitrogen over them, or you can freeze your tubes at -80oC the night before, put your aliquots in, and stick them back at -80oC.
Inoue Method and Inoue Method Derivatives
- There is an Inoue purist method posted on OpenWetWare.
- Frances Chu's post-doc lab swears by this method, or at least an extremely similar one.
- Some protocols claim to have improved that method.
- Example: UCSD version
- References within:
- High efficiency transformation of Escherichia coli with plasmids. Hiroaki Inouea, Hiroshi Nojimab, Hiroto Okayama, Gene 1990.
- The optimization of preparations of competent cells for transformation of E. coli. Tang 1994, NAR.
- Shows an interesting trend of competence versus OD when cells were harvested, and is reproducible across strains.
- Growth of Escherichia coli on Medium Containing Glycine Increases Transformation Efficiency. Akhtar MK1, Kaderbhai N, Kaderbhai MA. 2000. Analytical Biochemistry.
- "supplementation of glycine at 1% (w/v) in the growth medium of E. coli selectively interferes with the biosynthesis of cell wall by acting as a structural analog of L- and D-alanine found in the peptide units of peptidoglycan. Consequently, glycine causes synthesis of a defective but a "leaky" or more permeable cell wall, allowing selective discharge of periplasmic contents."
- References within:
- Example: UCSD version
Amanda's Protocol for Chemically Competent Cells
Supplies needed:
- Plate of E. Coli colonies
- SOB-Mg growth medium
- "-" is "minus", indicating that there is no added Mg
- Sterilized 500 mL Erlenmeyer flasks
- 50 mL screw-cap polypropylene tubes
- Freezer tubes (1.5 mL)
- SOC medium for recovery after heat shock (LB/TB is fine instead)
Method
- Pick several colonies off a freshly streaked plate into ~1 mL SOB-Mg growth medium
- Grow cells overnight or several hours in media with appropriate antibiotics if available.
- Use more inoculum in the next step if cultures weren't grown overnight.
- Inoculate 50 mL SOB-Mg growth medium with this culture. Use 500 uL of stationary phase culture for 50 mL SOB-Mg medium.
- Incubate at 275 rpm, 37oC until OD600 is about 0.3, which corresponds to ~ 5*107cells/mL
- Higher OD isn't usually a problem for routine work.
- Collect in sterile 50 mL polypropylene centrifuge tube(s) and chill on ice for 10 minutes
- Pellet the cells at 750 - 1,000g (2500 rpm) for 14 min at 4oC. Decant the supernatant and invert tubes to remove excess culture medium.
- Disperse cells in ~1/3 volume of CCMB by gentle vortexing or rapping of the centrifuge tube.
- Incubate on ice for 20 minutes
- Centrifuge at 2500 rpm for 10 min at 4oC
- Resuspend cells in CCMB at 1/12 the original culture volume
- Make aliquots in eppendorf tubes, ideally on ice
- Flash freeze with liquid nitrogen
- Store at -80oC to preserve them for many months
Recipes:
SOB-Mg growth medium (1 Liter)
| Ingredient | Amount |
|---|---|
| Bacto Tryptone | 20g |
| Bacto Yeast Extract | 5g |
| 1M NaCl | 10mL |
| 1M KCl | 2.5mL |
- Add water to make 1L
- Autoclave
- Dispense into smaller bottles for lower contamination risk
CCMB (1 Liter)
| Ingredient | Amount | Final Concentration |
|---|---|---|
| Potassium Acetate, 1M, pH 7 | 10 mL | 10mM |
| Glycerol | 100g | 10% (w/v) |
| CaCl2.2H2O | 11.8g | 80mM |
| MnCl2.4H2O | 4g | 20mM |
| MgCl2.6H2O | 2.5mL | 10mM |
- Prepare a 1M solution of potassium acetate, pH 7.0 using KOH.
- Filter through a 0.2 uM membrane & store frozen
- Prepare a solution of 10% potassium acetate, 10% glycerol
- Add salts, allowing each to enter solution before adding the next.
- Adjust pH to 6.4 with 0.1M HCl. Do not adjust pH upward with base.
- Filter through a 0.2 uM filter & store at 4oC.
Nicole/Andrew protocol for Chemically Competent cells
Materials and reagents
- E. coli line (Top 10, S17-1, BL21-AL, BL21-D3, JM109, Qiagen)
- TFB I (transformation buffer)
- TFB II
- TFB I (100 ml)
- 30 mM acetate K (0.294 g)
- 100 mM RbCl (1.21 g)
- 10 mM CaCl2 (0.14 g)
- 50 mM MnCl2 (1.0 g)
- 15% glycerol (15 ml)
- dH2O
- pH = 5.8 (use acetic acid to adjust)
- TFB II 100 ml
- 10 mM MOPS (0.21 g)
- 75 mM CaCl2 (1.1 g)
- 10 mM RbCl (0.12 g)
- 15% glycerol (15 ml)
- dH2O
- pH = 6.5 (use KOH to adjust)
Protocol
- 2 days before making cells, streak out the line of E. coli to make on LB plates (+strep for Top 10 and S17-1)
- 1 day before:
- inoculate 4 white capped test tubes or disposable 14 ml clear-top falcon tubes with 1 ml of LB (+strep)
- freeze appropriate color, autoclaved epi tubes in -80°C (80+ tubes)
- - white tube = Top 10
- - yellow tube = S17-1
- - pink tube = BL21-AL
- - purple tube = BL21-D3
- - green tube = JM109
- - blue tube = Qiagen
- Grow cells in 5 ml LB (+5 ul strep for Top 10 and S17-1 cells) overnight
- Transfer 1 ml of cells to each 50 ml LB flask and grow at 37°C for 90 min
- - want OD of 0.4 or 0.5 before starting next steps
- Place on ice (0°C) for 1 min
- Spin at 6000g, 0°C for 5 min
- Add 15 ml cold dH2O
- Spin at 6000g, 0°C for 5 min, pour off super
- Add 10 ml cold TFB I to pellet
- Incubate on ice for 15 min
- Spin at 6000g, 0°C for 5 min, pour off super
- Add 1 ml cold TFB II to pellet
- Incubate on ice for 30 min
- Aliquot 50 ul into -80°C epi tubes (or into tubes sitting in dry ice)
- Immediately store at -80°C
- Best method
- take tubes out of freezer
- open all caps
- pipette 50 ul into each
- close caps
- back in -80°C
- VERY QUICKLY!
TEST CELLS BEFORE STOCKING FOR GENERAL USE
- For contamination
- Scrape a sample from frozen stock
- Streak on LB (no abx)
- Grow at 37°C overnight
- Check for contamination (E. coli should be translucent and yellowish) – If none is present test competency
- For competency
- Use PCM184 plasmid stock (and Amp or Kan/Tet)
- Follow protocol for transformation
Electrocompetent Cells
- Reference protocols:
- You can easily make your own electro-competent cells for electroporation.
- Grow a small (~ 2 mL) overnight culture with appropriate antibiotic(s)
- Use overnight to inoculate: generally use ~ 200 uL/50 mL
- Let grow for about 3-4 h (OD 0.4-0.6 or so)
- If using the tube spec, the path length is longer than in the cuvettes. Divide tube specs by 1.65 to convert to the 1 cm path length OD. (If using tube spec, let the OD get to ~1.)
- Centrifuge and wash 2-3 times with 10% glycerol (everything on ice).
- Once or twice is fine. Don't waste time/energy doing much more. -JM 8/2013
- Remove pretty much all of the supernatant after the last centrifugation
- Leave just enough water to allow for pipetting. The volume of supernatant left should be < 1/500ths of the culture volume.
- See Janet's graph below. The really concentrated cells performed well despite having clumped into a serious "booger" during recovery (pre-plating) that was mostly unspreadable.
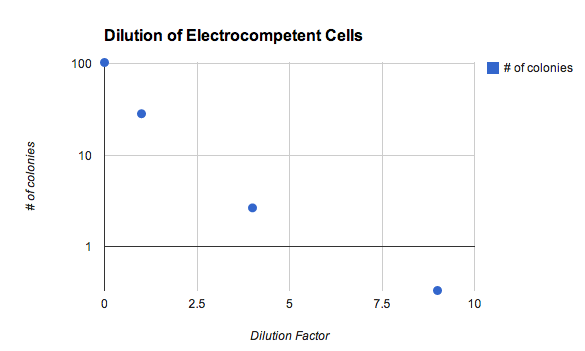
E. coli electroporation: number of colonies versus competent cell density. Note the log scale. - Each electroporation used 0.2 ng of pGA3K3 RFP DNA. (experiment details, experiment planning spreadsheet)
- Undiluted cells in this experiment are actually not very concentrated. Glycerol water of volume 1/500th of the culture volume was mixed back in. Janet has subsequently (8/2013) learned that more concentrated is much better, and is now routinely getting lawns from gibson assembly transformations.
- Aliquot into 1.5 ml centrifuge tubes (40-50uL in each), then flash freeze with liquid nitrogen. Store at -80C.
- Note: you can dilute your re-suspension, measure OD, and calculate the cell concentration if you care. See this page.
- [[User:Janet B. Matsen|Janet's] cells clump into a "booger" during recovery (pre-plating) that was mostly unspreadable. The clumping is less of an issue if you don't centrifuge them before plating. For this reason I recover in 200 uL and plate all of it.