James C. Clements: Week 3
From OpenWetWare
Jump to navigationJump to search
Lab Journal Navigator
Terminology
- Dynamical System: "A system that changes over time according to a set of fixed rules that determine how one state of the system moves to another state." [1]
- Law of Mass Action: "The law that states the following principle: the rate of a chemical reaction is directly proportional to the molecular concentrations of the reacting substances" [2]
- Homeostasis: "The ability of a system or living organism to adjust its internal environment to maintain a stable equilibrium; such as the ability of warm-blooded animals to maintain a constant temperature." [3]
- Equilibrium: "The condition of a system in which competing influences are balanced, resulting in no net change." [4]
- Oscillation: "A regular periodic variation in value about a mean" [5]
- Autocatalysis: "Catalysis in which the catalyst is one of the products of the reaction" [6]
Applying the Law of Mass Action
- Construct differential equations that model the following reactions. Be sure to define your state variables and rate constants.
- A + B → C
- A + B ↔ C
- A + B ↔ 2C
- 2A + 3B ↔ C+D
Please note that the symbol ↔ is used to denote arrows (reactions) in both directions.
Simulating Reaction Kinetics
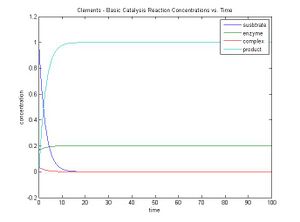
- Use the matlab code provided at my lionshare folder to study the simple reaction
- E + S ↔ ES → E + P
that we have studied in class. Set the parameters as follows:
- [S0] = 1.0
- [E0] = 0.2
- [ES0] = 0.0
- [P0] = 0.0
- k1 = 2.0
- k-1 = 0.0
- k2 = 10
Plot the output, and save the plot as an image. Post the image on the wiki as the answer to the question.