IGEM:Peking/2007/Count-Conjugation-Notebook/2007-8-11
From OpenWetWare
Jump to navigationJump to search
Tandem-OriT by Qu Mingzhi
OUR FIRST Biobricks !!
- F-OriT-J23066-OriT pSB1A2 now named as I741051!
- waiting for sequencing to get the final detail.
re-do: PlacI -> I741051
- basic experiment condition :IGEM:Peking/2007/Count-Conjugation-Notebook/2007-8-10
- we changed "fast T4 ligase" ligation time : from 20min ->10 min @16℃
- digesting for longer time.
- use both "fast T4 ligase" & normal T4 ligase".
electrophoresis result
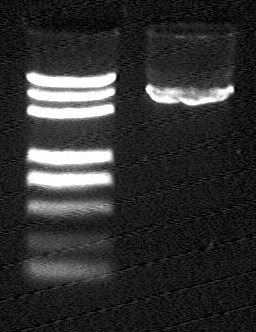
I741051 vector
- From left to right:
- Maker (DL2000 Plus)
- I741051 vector (after Gel extraction, EcoRI/XbaI)
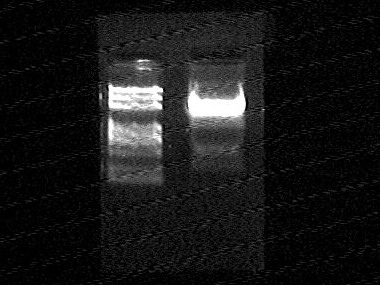
R0010 before gel extraction
- From left to right:
- R0010 -1 @ EcoRI/SpeI
- R0010 -2 @ EcoRI/SpeI
- Maker (DL2000 Plus)
R0010 after gel extraction
- R0010
- Maker (DL2000 Plus)
after ligation & mini-prep
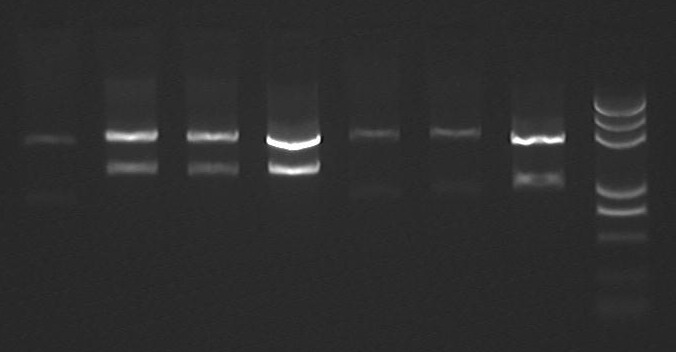
NEXT DAY(8-12):
- From left to right:
1,4,5 PlacI-I741051 @ EcoRI/PstI 2,3,4 J61003 @ EcoRI/PstI 7 I741051 @ EcoRI/PstI
oriT Knock Out
- By Xu Anting
Transformation of pKO3-oriT-deleted plasmid into DH5a
- Result: negative controls have an approximately number of clones in comparing with ligation products. It seems that our DH5a have been contaminated.
Ligation Products Verification
- By Colony PCR
- Picked colonies and grew them in an additional Cm Petri-dish, in 37 centrigrade, overnight.
Lock & Key By Yu Tao
Transformatiion Test of the Newly Prepared Competent Cells (Competent Cells I)
Transformation Result
- Number of colonies:
- Previous competent cells: more than 2000 clones.
- Newly prepared competent cells: fewer than 1000 clones.
- Comments: I think the efficiency of the new cells is a little bit lower and I decide to make some more competent cells tomorrow.
J01010 and J01008 (both by PCR) Digestion Product Purification
- Both of the fragments are digested from yesterday.
- use Transgen EasyPure PCR Purification Kit / Quick Gel Extraction Kit.
- 50uL per tube after purflication, one tube, respectively.
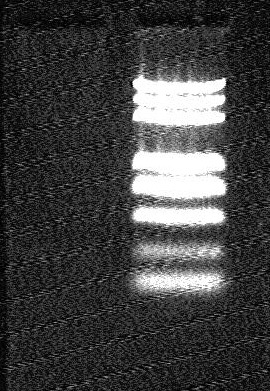
Electrophorsis Result
- from left to right:
- J01010 @ XbaI/PstI (2uL)
- J01008 @ XbaI/PstI (2uL)
- DL plus 2000 marker
- J01010 PCR product (2uL)
- J01010 PCR product (2uL)
- J01008 PCR product (2uL)
- DL plus 2000 marker
- marker
Ligation: R0010<-J01008 and R0040<-J01010
- Ligate the J01008 fragment and R0010 vector, also the J01010 fragment and R0040 vector.
- I use the previous digested R0040 @ PstI/SpeI and R0010 @ PstI/SpeI.
Electrophorsis Result
- from left to right:
- R0040 @ PstI/SpeI (Use PCR purification kit)
- R0010 @ PstI/SpeI (Use PCR purification kit)
- DL plus 2000 marker
- marker
- Comments: The R0040 @ PstI/SpeI seems degraded
- Ligation system contains:
8 µl J01008 fragment / 6.5 µl J01010 fragment 0.5 µl R0010 vector / 2 µl R0040 vector 0.5 µl Super T4-Ligase 1 µl 10 X ligation buffer -------------------------- 10 µl Total
- The negative control group contains no fragment but the same volume ddH2O instead.
- 10min at 16℃
Transformation: the new ligation product above
- Transform each 10 µl ligation system into 100 µl DH5α competent cells.
- Culture R0010<-J01010 cells at Amp+ and R0040<-J01008 LB plate for 12 hours.
- Result to be seen tomorrow.