IGEM:IMPERIAL/2008/Prototype/Project
<html> <style type="text/css"> .firstHeading {display: none;} </style> </html> <html> <style type="text/css">
table.calendar { margin:0; padding:2px; }
table.calendar td { margin:0; padding:1px; vertical-align:top; } table.month .heading td { padding:1px; background-color:#FFFFFF; text-align:center; font-size:120%; font-weight:bold; } table.month .dow td { text-align:center; font-size:110%; } table.month td.today { background-color:#3366FF } table.month td {
border:2px; margin:0; padding:0pt 1.5pt; font-size:8pt; text-align:right; background-color:#FFFFFF; }
- bodyContent table.month a { background:none; padding:0 }
.day-active { font-weight:bold; } .day-empty { color:black; } </style> </html>
| <html><a href=http://openwetware.org/wiki/IGEM:IMPERIAL/2008/Prototype><img width=50px src=http://openwetware.org/images/f/f2/Imperial_2008_Logo.png></img</a></html> | Home | The Project | B.subtilis Chassis | Wet Lab | Dry Lab | Notebook |
|---|
<html> <style type="text/css"> div.Section { font:11pt/16pt Calibri, Verdana, Arial, Geneva, sans-serif; }
/* Text (paragraphs) */ div.Section p { font:11pt/16pt Calibri, Verdana, Arial, Geneva, sans-serif; text-align:justify; margin-top:0px; margin-left:30px; margin-right:30px; }
/* Headings */ div.Section h1 { font:22pt Calibri, Verdana, Arial, Geneva, sans-serif; text-align:left; color:#3366FF; }
/* Subheadings */ div.Section h2 { font:18pt Calibri, Verdana, Arial, Geneva, sans-serif; color:#3366FF; margin-left:5px; }
/* Subsubheadings */ div.Section h3 { font:16pt Calibri, Verdana, Arial, sans-serif; font-weight:bold; color:#3366FF; margin-left:10px; }
/* Subsubsubheadings */ div.Section h4 { font:12pt Calibri, Verdana, Arial, sans-serif; color:#3366FF; margin-left:15px; }
/* Subsubsubsubheadings */ div.Section h5 { font:12pt Calibri, Verdana, Arial, sans-serif; color:#3366FF; margin-left:20px; }
/* References */ div.Section h6 { font:12pt Calibri, Verdana, Arial, sans-serif; font-weight:bold; font-style:italic; color:#3366FF; margin-left:25px; }
/* Hyperlinks */ div.Section a {
}
div.Section a:hover {
}
/* Tables */ div.Section td { font:11pt/16pt Calibri, Verdana, Arial, Geneva, sans-serif; text-align:justify; vertical-align:top; padding:2px 4px 2px 4px; }
/* Lists */ div.Section li { font:11pt/16pt Calibri, Verdana, Arial, Geneva, sans-serif; text-align:left; margin-top:0px; margin-left:30px; margin-right:0px; }
/* TOC stuff */ table.toc { margin-left:10px; }
table.toc li { font: 11pt/16pt Calibri, Verdana, Arial, Geneva, sans-serif; text-align: justify; margin-top: 0px; margin-left:2px; margin-right:2px; }
/* [edit] links */ span.editsection { color:#BBBBBB; font-size:10pt; font-weight:normal; font-style:normal; vertical-align:bottom; } span.editsection a { color:#BBBBBB; font-size:10pt; font-weight:normal; font-style:normal; vertical-align:bottom; } span.editsection a:hover { color:#3366FF; font-size:10pt; font-weight:normal; font-style:normal; vertical-align:bottom; }
- sddm {
margin: 0; padding: 0; z-index: 30 }
- sddm li {
margin: 0; padding: 0; list-style: none; float: center; font: bold 12pt Calibri, Verdana, Arial, Geneva, sans-serif; border: 0px }
- sddm li a {
display: block; margin: 0px 0px 0px 0px; padding: 0 0 12px 0; background: #33bbff; color: #FFFFFF; text-align: center; text-decoration: none; }
- sddm li a:hover {
border: 0px }
- sddm div {
position: absolute; visibility: hidden; margin: 0; padding: 0; background: #33bbff; border: 1px solid #33bbff } #sddm div a { position: relative; display: block; margin: 0; padding: 5px 10px; width: auto; white-space: nowrap; text-align: left; text-decoration: none; background: #FFFFFF; color: #2875DE; font: 11pt Calibri, Verdana, Arial, Geneva, sans-serif } #sddm div a:hover { background: #33bbff; color: #FFFFFF } </style></html>
<html><a href=http://openwetware.org/wiki/IGEM:IMPERIAL/2008/Prototype/Motility><img width=200px src=http://openwetware.org/images/4/42/Imperial_2008_Motility_Label.png></img></a></html> |
 |
<html><a href=http://openwetware.org/wiki/IGEM:IMPERIAL/2008/Prototype/Biomaterials><img width=200px src=http://openwetware.org/images/0/04/Imperial_2008_Biomaterials_Label.png></img></a></html> |
<html><a href=http://openwetware.org/wiki/IGEM:IMPERIAL/2008/Prototype/Light><img width=200px src=http://openwetware.org/images/3/3d/Imperial_2008_Light_Label.png></img></a></html> |
Given below is an overview of B. subtilis; why we chose it as the chassis for our project and what makes it a useful organism for Synthetic Biology (and iGEM in particular!). In addition, there is a regularly-updated "Progress & Problems" section along with information about the distribution of our team.
B. subtilis
B.subtilis as a chassis made its first appearance last year and has many advantages for use at iGEM including (but not limited to!) a single membrane allowing for easier secretion, its high motility and a large amount of data in the literature. For our project, B.subtilis is vital. E.coli cannot secrete proteins to any high degree and modifications to it to allow this have not always proven particularly succesful. B.subtilis has however been used for this purpose for many decades and though there are some technical issues associated with this, it has performed the job admirably. B.subtilis is also highly motile and posses a unique (at present) system of switching off its motility in the form of a molecular clutch, EpsE (see Motility below).
To make B.subtilis a viable host, it is necessary to produce parts that will work correctly in B.subtilis. When searching the literature, it rapidly became clear that B.subtilis would not be able to use E.coli promoters with any real ability, would happily digest many E.coli produced vectors and also lacked a Ribosome capable of binding Ribosome Binding Sequences from E.coli. As such, we would need new vectors, new promoters and new RBSs in order to get the project off the ground.
To get round modifying a shuttle vector to meet Biobrick standards integration sequences can be used. These sequences are complementary to a gene in B.subtilis and may naturally be incorporated over those genes by the recombination system in B.subtilis. Promoters for B.subtilis were taken from both vectors and the organisms own chromosome via the extensive transcription regulation database for B.subtilis, the DBTBS, to suit the requirements of our system and to give a suitable range of basic promoters for B.subtilis. Although a consensus sequence (5'-AAGGAGGTG-3') for the B.subtilis RBS exists and has been proven to work effectively in the organism, two naturally occuring RBSs from B.subtilis that have been used extensively in the past were chosen as these are the most likey to work.
Progress so Far
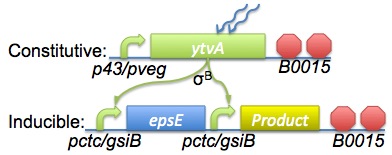
The diagram on the right (click for larger image) shows a theoretical overview of the circuitry we'll be adding to B. subtilis in order to control its movement and expression profile.
The top row shows synthetic constructs which we will add that are to be constitutively expressed and the bottom row shows synthetic constructs that we require to be inducible. Each gene is preceded by its own promoter/RBS pair; this is to reduce the impact of the reduction in efficiency which occurs for each successive gene in a row during B. subtilis transcription.
The general operation of these constructs should fulfil our specifications, as the simple explanations below demonstrate...
- No light present: Inducible promoter is not activated, meaning no transcription of product and no production of synthetic EpsE. The cell's own background expression of SinR should prevent production of native EpsE so the bacteria will remain motile. If the native SinR is found to be ineffective in this capacity, we may integrate this construct over the top of the cell's native epsE gene - effectively knocking it out.
- Light present: Inducible promoter is activated, meaning product is transcribed along with EpsE. Native EpsE is still repressed by the native SinR, but the synthetic EpsE should engage the clutch and halt the bacteria in a short timeframe. The bacteria should, therefore, stop and start producing product.
- Light removed: Should a bacteria move out of the light, we want it to be able to turn off production and start moving again. When light is absent, the inducible promoter will no longer be active and the bacteria should revert to the "no light" state. The time it takes to do this will be directly linked to the accuracy of deposition, and so we want it to be as fast as possible.
We are currently in the preliminary wetlab stages of the project, having designed and sent of the sequences for our parts to Geneart for synthesis. We have constructed a critical pathway for the cloning steps to take us from or ordered constructs (plus a few Registry ones) to our finished test constructs - more information about this can be found on the wetlab page. We are currently in phase 0 of an outlined 4 - phase 0 using constructs from the Registry only, testing them in B. subtilis and designing basic devices that will be re-used later. Phase 1 will begin when we receive our synthesised constructs from Geneart.
Summary Presentation of work so far - Wet lab
Potential Issues
The constructs we have designed are polycistronic, B.subtilis translation of polycistronic genes however is not even. The first gene in a polycistron is often more highly expressed than the second even if they have their own individual Ribosome Binding Sites. This may cause issues for our current circuit design but could be easily remedied by using multiple promoters in each construct or by the arrangement of genes along the polycistron.
There is an issue with transcription of multiple genes in series in B. subtilis - with each successive gene, the efficiency of transcription is attenuated. This will require us to add the relevant promoter/RBS pair in front of each gene in a series.
We had intended to include with our constitutive expression construct a gene for SinR. SinR is the native repressor of the EpsE clutch mechanism, and over-expression would thus allow us more control over the motility of our bacteria. Additionally SinR downregulates several undesirable product pathways including protease-producing and competence-reducing operons. We found, however, that in the long run it also had undesirable effects that could interfere with our aims so we decided to leave it out. This does pose a problem; what if the native SinR is not enough to repress the native EpsE? Our bacteria will effectively maintain natural control of their motility. We believe this will not be the case, but if it turns out to be so we have formulated several workarounds:
- Using epsE-knockout strains of subtilis is one solution, but we would rather not have to use this as it's more a genetic engineering approach than a Synthetic Biology one
- Integrating over the epsE gene instead of the amyE gene is preferable - changing our integration sites such that, by transformation, we are effectively also disrupting the bacteria's natural EpsE production machinery
Team Divisions
At the moment we're separated into two teams:
- The wetlab team (James, Chris, Tom, Krupa & QQ) has so far concentrated on the mammoth task of finding sequences and writing protocols, and will move on to setting up and organising experiments.
- The drylab team (Erika, Prudence, Clinton & Yanis) is working out a strategy for modelling of behaviour of our bacteria on Matlab, and will be using data from a recorded microscope analysis of subtilis motility to help define parameters. More detail can be found on the Wet Lab and Dry Lab pages.