Griffin:Nested RT-PCR
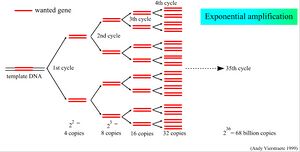
qPCR Advantages
- Collect reliable data at low cycle numbers. Traditional PCR is less sensitive/ must proceed to higher Ct observe on EtBr agarose gel.
- qPCR (real time) data are captured the duration of low cycle number and minimizes error.
- Earlier and more sensitive early cycle measurements in the amplification process mitigate substrate exhaustion (dNTPs).
Ct (threshold cycle) value
Ct (threshold cycle) value is cycle number at which fluorescence emission (SYBR Green) emerges from the fluorescence threshold. Fluorescence threshold signal represents an emission (enrichment) output above background fluorescence. At Ct (threshold cycle), amplicon product at a detectable amount is entering the early exponential phase of expansion.
- The lower a Ct value, the higher amount of starting substrate (parent template).
- Ct (threshold cycle) are inversely proportional to basal expression of target nucleic acid (mRNA)
- Ct (threshold cycle) minimum fluorescence detection sensitivity is reaents, primers, and qPCR apparatus dependent.
- The cycle number at threshold value detection =cycle threshold (Ct).
Ct <25 = positive/abundant mRNA Ct 25-30 = positive/moderate mRNA Ct 30-40 = negative/minimal mRNA/false positive/contamination
- SYBR Green alone on fluorescent plate reader at 497 nanometer blue light excitation fluoresces =background fluorescence.
- SYBR Green intercalates into dsDNA, and with robust fluorescence @(λmax = 497 nm) emits @ band pass (λmax = 520 nm).
- One unit of dsDNA to SYBR Green = one unit of fluorescence (after subtracting background fluorescence in excess amount of SYBR Green substrate). Two units of DNA = 2x as much fluorescence.
Traditional (Nested) PCR overview
Santa Cruz Biotechnology inc. offers nested primers for measuring transcript levels. Each product will have 2 vials, an A set (forward & reverse) and B set (forward & reverse) primers. Each set of primers is provided 20 µl at a concentration of 10 µM. Since the primers are designed toward an mRNA template, they are designed to cross exon junctions and will not amplify genomic DNA.
The amplification of RNA requires the conversion of the RNA substrate into DNA. This is achieved through the use of a reverse transcriptase such as AMV RT (avian myeloblastis virus reverse transcriptase) or M-MuLV RT (moloney murine leukemia virus reverse transcriptase). The resulting cDNA can be used as a template for a standard PCR.
Nested PCR is a strategy where two pairs of PCR primers (forward and reverse) target a single locus with a B set 'nested' within an A set. The initial amplification pair (A set) generates an ~1kb amplicon off (total RNA /RT derivative) cDNA. The second amplification pair (B set; nested pair) target within the first amplicon, and produce a secondary ~500bp amplicon. The probability of reaching specific B set logarithmic amplification is dependent on an initial A set dependent amplification.
A set primer amplicon size ~1000 bp
B set primer amplicon size 250-500 bp
- To identify low levels of DNA contamination, do a PCR of a housekeeping gene and a portion of the RNA preparation as template. If there is contamination, there will be products in all samples.
Primer Tm Values
Tm values for PCR primers range between 55-60 C (19-22 nt, GC% ~55%, no Salt) OR 63-68 C w/salt. The A and B nested primer sets share similar base pair length, GC% and Tm values.
Nested PCR utilizes two pairs of PCR primers for a single locus. The first primer pair A set amplifies within the locus. The second primer pair B set (nested primers) then binds within the 'A' amplicon to produce a second nested 'B' amplicon.
Reagents
- Reverse Transcriptase
- Deoxynucleotide Mix, dATP, dCPT, dGTP, dTTP, 10 mM each, in sterile double-distilled water, pH 8.5
- Reaction Buffer, 10x conc., 1.05 ml; 100 mM Tris-HCl, 500 mM KCl, pH 8.3 (20°C)
- MgCl2 Stock Solution, 2 x 1.3 ml each 25 mM MgCl2
- Gelatin, 0.05% gelatin (w/v)
- Oligo-p(dT)15 Primer, 0.02 A260 units/µl (0.8 µg/µl)
- RNase Inhibitor, 50 U/µl
- Sterile Water
Oligo-p(dT)15 Primer is a conventional primer component that binds polyAdenylation tracts on protein coding transcripts (mRNA) as an integral part of a reverse transcription reaction (with reverse transcriptase)
- Oligo-p(dT)15 Primer, 0.02 A260 units/µl (0.8 µg/µl)
- Each vial contains 40 µg in 8 nmol of PBS with < 0.1% sodium azide and 0.1% gelatin.
- Product as 40 ug of lyopholized powder store at -20 C.
- Reconstitute with 50 uL (0.8ug/uL) of double distilled water (also store at -20 C).
- Prepare aliquots of the reconstituted form/ avoid repeated freeze and thaw cycles.
PCR Optimization
- MgCl2 concentrations may vary depending on the template, primer, and dNTP concentrations in the amplification reaction. To optimize conditions, use a MgCl2 titration, generally between 0.5 and 10 mM.
- Primer concentrations may vary; typical final concentrations range from 0.01 to 0.5
- The amount of cDNA utilized in RT-PCR reactions may vary depending on the nature of the RNA template; typically, 5 ul of the cDNA of reverse transcribed total RNA, 20 ul of the cDNA resulting from reverse transcribed poly(A)+ RNA, or 20
- Addition of Gelatin (0.01 mg/ml final) stabilizes Taq DNA polymerase during the PCR reaction, yielding more amplification product.
- RNA quality Assessment
- Proposal: gDNA contamination (relative quantification) is acceptable if gDNA amplifies >5 cycles after the cDNA amplification (>32 fold less template).
DNA Contamination
Non-specific amplicons can arise from primer-dimer formation or unspecific background amplification of genomic (gDNA). Primers toward an existing transcript or RNA entity control for false genomic DNA (gDNA dependent) amplicon detection, when reaction conditions yield true (mRNA dependent) amplicon detection.
Housekeeping genes for normalization of transcript (gene expression) data can be suitable for gDNA contamination controls in qRT-PCR experiments.
- 18s rRNA
- 18S rRNA is proposed to be predominately absent poly(Adenylated) tail, and therefore should not appear to amplify in an Negative Reverse Transcriptase (-RT) control PCR reaction.
-/+ Reverse Transcriptase
RNA quality (purity and integrity) and quantity are critical to reliable gene expression analysis.
Negative Reverse Transcriptase (-RT) as a control when preparing cDNA from an RNA sample extraction preparation.
Reaction 1 (-RT) = Negative RT Control = RNA (DNase treated) + dNTPS + Random Hexamers/Oligo dT + RT Buffer + Water // NO Reverse Transcriptase//. Reaction 2 (+RT )= WITH Reverse Transcriptase (cDNA from polydt)
Perform PCR with housekeeping primers.
No gDNA contamination / -RT will be negative (no amplicon) / +RT will be positive YES gDNA contamination/ -RT will be positive (yes amplicon); incomplete DNase digestion of the RNA prep.
Performing amplification of a sample prior to reverse transcriptase / minus reverse transcriptase control (no reverse transcriptase) determines to what extent genomic DNA contamination exists from the RNA prep. Determination of DNA contamination present in an RNA preparation controls for false positive qPCR. Amplification of traditional housekeeping genes (ie GAPDH) within a RNA prep (no RT) suggests gDNA contamination.
DNase digestion
DNase digestion, for primers that do not span exon/exon boundaries (ie GAPDH), one proposal is to digest RNA prior to RT (1 µg RNA + 1 unit DNase I, 37°C 45 min).
cDNA Synthesis (Reverse Transcription)
In a standard RT-PCR assay, varying amounts of RNA template 10ug, 1ug, 100ng, 100pg are reversely transcribed with a poly dT primer that attaches to the polyadenylation track on mRNA to yield a cDNA template.
- Prepare a solution containing:
a) 1 ul oligo (dT)12–18 (500 ug/ml)
b) 1 ng-5 ug total RNA
c) 1 ul 10 mM dNTPs
d) and add RNase-free water to a final volume of 12 ul
- If extensive secondary structure is potentially present in the RNA, the RNA sample may be denatured at +70°C for 5 min before adding it to the reaction minimize RNA secondary structure, and placed on ice for 5 min before adding it to the reaction.
a) 4 ul 5x reverse transcriptase buffer
b) 2 ul 0.1 M DTT
c) 1 u RNase inhibitor
Reverse Transcritpion Reaction
- Incubate at +30°C for 10 min minutes to anneal primer and template.
- Add 1 ul reverse transcriptase (200 units) and incubate at 42° C for 60 minutes to extend the primer and then terminate the reaction by incubating at 70° C for 15 minutes. The RNA is subsequently reverse transcribed, resulting in cDNA synthesis.
NOTE: (As an optional step add 1 ul RNase H (2 unit/ul) and incubate at 37° C for 20 minutes)
First PCR reaction
- Prepare a solution containing:
a) 5 ul 10x PCR buffer (with or without MgCl2)
b) 5 ul 25 mM MgCl2 (It may be necessary to vary the MgCl2 concentration, 2.5 mM final concentration recommended.)
c) 1 ul 10 mM dNTP
d) 1 ul primer pair A
e) 1 ul Taq DNA polymerase
f) 2 ul cDNA and add water to 50 ul
First PCR amplification parameters
Perform 15–35 cycles of PCR. Annealing and extension conditions are primer and template dependent and must be determined empirically for each template-primer pair. Tm values for PCR primers generally range between 55-60 C
- Denaturation 96°C, 0.5-1.5 minute
- Annealing 57°C, 0.5-1 minute
- Polymerization (35 cycles) 72°C, 0.5-2 minutes
- Link; Extension (1 cycle) 70°C, 5 minutes
- Link to a 4°C Soak file.
NOTE: Addition of Gelatin (0.01 mg/ml final) stabilizes Taq DNA polymerase, yielding more amplification product.
Second (nested) PCR reaction
- Prepare a solution containing:
a) 5 ul 10x PCR buffer (with or without MgCl2)
b) 5 ul 25 mM MgCl2 (It may be necessary to vary the MgCl2 concentration, 2.5 mM final concentration recommended)
c) 1 ul 10 mM dNTP
d) 1 ul primer pair B
e) 1 ul Taq DNA polymerase
f) 1–5 ul first PCR product and add water to 50 ul
Second PCR amplification parameters
Perform 15-25 cycles of PCR. Annealing and extension conditions are primer and template dependent and must be determined empirically for each template-primer pair. Tm values for PCR primers generally range between 55-60 C
- Denaturation 96°C, 30 sec
- Annealing 57°C, 30 sec
- Polymerization (35 cycles) 72°C, 30 sec
- Link; Extension (1 cycle) 70°C, 5 minutes
- Link to a 4°C Soak file.
NOTE: Addition of Gelatin (0.01 mg/ml final) stabilizes Taq DNA polymerase, yielding more amplification product.
- PCR products are visualized by agarose gel electrophoresis stained with an appropriate dye.
Agarose Gel Electrophoresis
Agarose gel electrophoresis can resolve DNA or RNA by size. DNA/RNA is visible in the gel when ethidium bromide is added during the gel casting process. EtBr intercalates between dsDNA or dsRNA and absorbs invisible UV light and emits visible orange light. SYBR Green absorbs blue light (λmax = 488 nm) and emits green light (λmax = 522 nm) may be used in place of EtBr, although more commonly this dye is used for qPCR.
- 0.7% gel will show good separation (resolution) of large DNA fragments (5–10kb)
- 2% gel will show good resolution for small fragments (0.2–1kb)
- 3% gel or vertical polyacrylamide gel for separating smaller fragments (the higher the % gel, the more brittle the gel)
Gel Casting
The volume of agarose required for a minigel is around 30–50mL.
- Weigh out 0.5g of agarose into a 250mL conical flask. Add 50mL of 0.5xTBE, swirl to mix.
- Microwave for about 1 minute to dissolve the agarose. Use gloves to handle and do not let it overboil; molten agarose is very hot.
- Let cool for 5 minutes or warm to touch with bare hands.
- Add 1µL of ethidium bromide (10mg/mL) and swirl to mix.
- Pour the gel into the resevoir. Push any bubbles away to the side using a disposable tip. Insert the comb. Wait at least 30-60 min.
- Add 0.5x TBE buffer into the gel tank to submerge the gel to 2–5mm depth. This is the running buffer.
- Load samples and run the gel no greater than 5 Volt/cm.
2L of 10xTBE
- 218g Tris base
- 110g Boric acid
- 9.3g EDTA
Dissolve the ingredients in 1.9L of distilled water. pH to about 8.3 using NaOH and make up to 2L.
Loading buffer
- 25mg bromophenol blue or xylene cyanol (Bromophenol blue migrates @ ~200–400bp. Xylene cyanol migrates ~4kb)
- 4g sucrose
- H2O to 10mL
Store at 4°C or -20
References
Sambrook, J., Fritsch, E.M. and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Edition, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, pp. 14.16, 14.20.