Griffin:Flow Cytometry prep & labeling
Spectral Overlap
Fluorescent Proteins
- Excitation/emission (nm): 558/583 (RFP excites @558 nm laser/emits 585+/-8nm (similar to PE 565nm/575nm)).
- Origin: Discosoma sea anemones.
GFP Green Fluorescence Proteins
GFP Origin: Aequoera victoria jellyfish
- Excitation/emission (nm): 395/510
CopGFP Origin: Pontellina plumata
- Excitation/emission (nm): 488/509
- CopGFP is a novel fluorescent protein from copepod plankton similar to EGFP / brighter color.
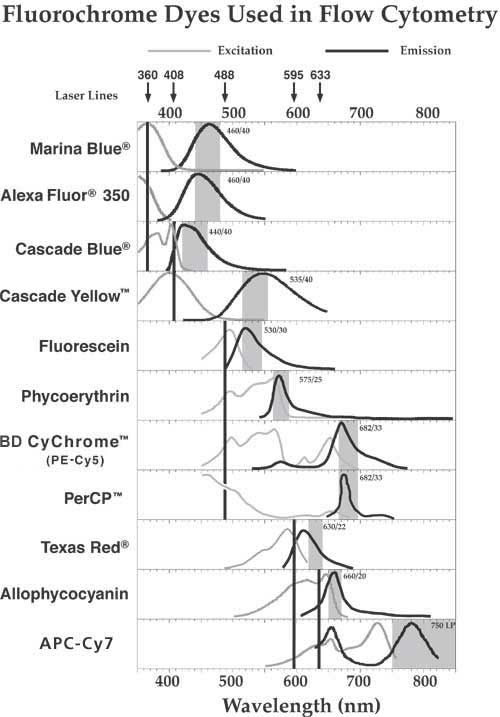
Excitation / Emission
- Spectra Table
- Alexa Fluor Excitation/Emission
- Alexa Fluor
- California Red________excitation/emission (nm) : 592/609 nm. Excite @561 nm laser /582/15 nm bandpass. California Red™ is a superior replacement for Texas Red®
- Cyanine-3(CY3)________excitation/emission (nm) : 555/569
- Cyanine-5(CY5)________excitation/emission (nm) : 651/670
- FITC__________________excitation/emission (nm) : 494/520
- TRITC_________________excitation/emission (nm) : 544/570 Excite @561 nm laser /586/15 nm (aka tetramethylrhodamine B isothiocyanate)
- Phycoerythrin(PE)_____excitation/emission (nm) : 565/575
- Rhodamine_____________excitation/emission (nm) : 541/572
- Texas Red_____________excitation/emission (nm) : 596/620
- APC (Allophycocyanin)_excitation/emission (nm) : 651/660; (640 nm laser @ 670/30 nm bandpass)
- PerCP (Peridinin chlorophyll) excitation/emission (nm): 477nm/678nm
- PerCP-Cy5.5___________excitation/emission (nm) : 482/676
- PerCP-Cyanine5.5 dye__excitation/emission (nm) : 482/676 near-IR fluorophore
- PE-Cy7________________excitation/emission (nm) : 496/785
Alexa Fluor
- Alexa Fluor 405 ~1028.3 g/mol Ex401 Em421
- Alexa Fluor 488 ~643.4 g/mol Ex496 Em519
- Alexa Fluor 546 ~1080.4 g/mol Ex556 Em573
- Alexa Fluor 555 ~534.5 g/mol Ex555 Em565
- Alexa Fluor 594 ~819.9 g/mol Ex590 Em617
- Alexa Fluor 647 ~1025.2 g/mol Ex650 Em665
- Alexa Fluor 680 ~1150 g/mol Ex679 Em702
- Alexa Fluor 790 ~1750 g/mol Ex784 Em814
CruzFluor™ Excitation/Emission
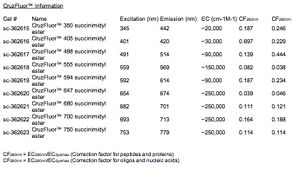
- CruzFluor™ 350: Ex (nm) 345 Em (nm) 442
- CruzFluor™ 405: Ex (nm) 401 Em (nm) 420
- CruzFluor™ 488: Ex (nm) 491 Em (nm) 514
- CruzFluor™ 514: Ex (nm) 518 Em (nm) 542
- CruzFluor™ 532: Ex (nm) 542 Em (nm) 558
- CruzFluor™ 555: Ex (nm) 559 Em (nm) 569
- CruzFluor™ 594: Ex (nm) 592 Em (nm) 614
- CruzFluor™ 633: Ex (nm) 638 Em (nm) 655
- CruzFluor™ 647: Ex (nm) 654 Em (nm) 674
- CruzFluor™ 680: Ex (nm) 682 Em (nm) 701
- CruzFluor™ 700: Ex (nm) 693 Em (nm) 713
- CruzFluor™ 750: Ex (nm) 753 Em (nm) 779
- CruzFluor™ 790: Ex (nm) 782 Em (nm) 811
Lyse Red Blood Cells
NOTE: This will prepare enough cells for 10 samples @ 100ml/test.
- Add 1ml of whole blood to 14ml of RT 1X Ammonium Chloride lysing solution.
- Vortex gently and incubate @ RT for 3-5 minutes. Do NOT exceed 5 minutes.
- Centrifuge for 5 minutes, aspirate supernatant, and gently re-suspend pellet in 5ml of cold 1X PBS.
- Centrifuge again for 5 minutes, aspirate supernatant, and gently re-suspend pellet in 1ml of cold 1X PBS.
Cell Surface Staining
- Label tubes.
- Add fluorochrome-conjugated antibody to tubes. (Use 1/0.5mg per 10e6 cells.)
- Add 100ml of RBC-lysed blood to tubes.
- Vortex and incubate for 15-30 minutes in a covered ice bucket.
- Add ~1.5ml of wash buffer (1X PBS) to each tube and mix by inversion.
- Centrifuge tubes @ 1000RPM for 5 minutes, aspirate supernatant, and resuspend pellet in 500ml of 1% paraformaldehyde.
Acquire and Analyze Data
Alternately, a lyse/wash procedure can be used in which whole blood is stained, then treated with a “Flow Lysis Buffer” and washed. This “Flow Lysis Buffer” is different from the Ammonium Chloride lysing solution.
Indirect Immunofluorescence Labeling
Lyse Red Blood Cells
NOTE: This will prepare enough cells for 10 samples @ 100ml/test.
- Add 1ml of whole blood to 14ml of RT 1X Ammonium Chloride lysing solution.
- Vortex gently and incubate @ RT for 3-5 minutes. Do NOT exceed 5 minutes.
- Centrifuge for 5 minutes, aspirate supernatant, and gently re-suspend pellet in 5ml of cold 1X PBS.
- Centrifuge again for 5 minutes, aspirate supernatant, and gently re-suspend pellet in 1ml of cold 1X PBS.
Cell Surface Staining
- Label tubes.
- Add unconjugated primary antibody to tubes. (Use 1-0.5mg per 10e6 cells.)
- Add 100ml of RBC-lysed blood to tubes.
- Vortex and incubate for 15-30 minutes in a covered ice bucket.
- Add ~1.5ml of wash buffer (1X PBS) to each tube and mix by inversion.
- Centrifuge tubes @ 1000RPM for 5 minutes, aspirate supernatant, and re-suspend pellet in 100ml of 1X PBS.
- Add fluorochrome-conjugated secondary antibody to tubes. (Use 1-0.5mg per 10e6 cells.)
- Vortex and incubate for 15-30 minutes in a covered ice bucket.
- Add ~1.5ml of wash buffer (1X PBS) to each tube and mix by inversion.
- Centrifuge tubes @ 1000RPM for 5 minutes, aspirate supernatant, and re-suspend pellet in 500ml of 1% paraformaldehyde.
Acquire and Analyze Data
Staining Intracellular Cytokines
Surface Staining
- Label tubes
- Add fluorochrome-conjugated surface-antigen specific antibodies to tubes. be sure to do pairs: unstimulated and activated.
- Add unstimulated or activated blood to appropriate tubes. Mix well and incubate for 15-30 minutes @ RT in the dark. Keep in mind that some antibodies with a low antigen density may require longer staining times.
- Lyse RBC’s (2ml of Lysing buffer per 100ml of whole blood). Incubate @ RT in the dark for 10 minutes. Do not exceed 15 minutes.
- Centrifuge samples (300-400xg) @ RT, aspirate supernatant, wash once with staining buffer (PBS/Azide + BSA).
Fixation and Permeabilization
- Immediately after the wash step, fix the cells by adding 100ml of fixation solution while vortexing the tube and incubate in the dark @ RT for 20 minutes.
- Add 1ml of Permeabilization buffer to each tube, centrifuge for 5 minutes, aspirate supernatant, repeat once.
4) Intracellular Staining
- Dilute the fluorochrome-conjugated anti-cytokine antibody in Permeabilization buffer and add to the appropriate tube. (Use 0.5-0.06mg/10e6 cells.)
- Incubate in the dark @ RT for 20 minutes.
- Add 1ml of Permeabilization buffer to each tube, centrifuge for 5 minutes, aspirate supernatant.
- Resuspend the pellet in 0.5ml staining buffer.
Acquire and Analyze Data
Cell Activation
Lymphocyte Activation
for 5 samples each (unstim. & act.) @ 100ml/test
Component: Unstimulated/Activated
- RPMI-1640 (+2mM L-glutamine): 500ml/500ml
- Brefeldin A: 10mg/10mg
- PMA: --/25ng
- Ionomycin: --/1mg
Whole blood w/ heparin: 500ml/500ml
Monocyte Activation
for 10 samples each (unstim. & act.) @ 50ml/test
Component: Unstimulated/Activated
- Brefeldin A: 10mg/10mg
- LPS: --/1mg
- Whole blood w/ heparin: 1ml/1ml
Incubate @ 37°C, 7% CO2 for 4 hours.
Cell Detachment
EDTA
EDTA solution chelates calcium, is less stringent than trypsin, and may help preserve ECM epitopes.
- 10mM EDTA (<15mM EDTA). Pipet EDTA solution into flask/plate and incubate for 1 minute. Gently tap the flask to determine cell adherence. Allow to incubate at 37C up to 10 minutes. Increase EDTA mM concentration according to strength of adherent cell.
Citric Saline
- 500mL 10x solution: 50.3 g KCl, 22.06 g Sodium Citrate.
Pipet prewarmed 1X Citric Saline solution into flask/plate and incubate at 37C for 4 minutes. Remove cells be gently tapping or pipetting up and down several times.