Biomod/2012/HKBU/BU Magician:Results
<html> <style> .container{background-color: #FFFFFF; margin-top:0px}
- column-content {width: 0px; float: left; margin: 0 0 0 0;padding: 0;}
.firstHeading {display:none; width:0px;}
- column-one {display:none; width:0px;background-color: #FFFFFF;}
- globalWrapper{width: 920px; background-color: #FFFFFF; margin-left: auto;margin-right: auto;
-moz-box-shadow: 0px 0px 15px #ccc; -webkit-box-shadow: 0px 0px 15px #ccc; box-shadow: 0px 0px 15px #ccc;}
- content{ margin: 0 0 0 0; align: center; padding: 12px 12px 12px 12px; width: 800px;background-color: #FFFFFF; border: 0;}
- bodyContent{ width: 800px; align: center; background-color: #FFFFFF;}
- column-content{width: 800px;background-color: #FFFFFF;}
</style> </html> <html xmlns="http://www.w3.org/1999/xhtml" xml:lang="en" lang="en"> <head> <meta http-equiv="Content-Type" content="text/html; charset=UTF-8" /> <title>jOuery</title> <link rel="stylesheet" href="style.css" /> <style> .menu { height: 45px; display: block; } .menu ul { list-style: none; padding: 0; margin: 0; } .menu ul li { float: left; overflow: hidden; position: relative; text-align: center; line-height: 45px; } .menu ul li a { position: relative; display: block; width: 128px; height: 45px; font-family: Arial; font-size: 11px; font-weight: bold; letter-spacing: 1px; text-transform: uppercase; text-decoration: none; cursor: pointer; } .menu ul li a span { position: absolute; left: 0; width: 128px; } .menu ul li a span.out { top: 0px; } .menu ul li a span.over, .menu ul li a span.bg { top: -45px; }
- menu2 { background:#45A8DF; }
- menu2 ul li a { color:#FFF; }
- menu2 ul li a span.over { background: #A6DD00; color:#333; }
- menu2 ul li.nav1 a span.over { background: #fea274; }
- menu2 ul li.nav2 a span.over { background: #b0bbba; }
- menu2 ul li.nav3 a span.over { background: #a3f091; }
- menu2 ul li.nav4 a span.over { background: #86dbf9; }
- menu2 ul li.nav5 a span.over { background: #e0caf0; }
- menu2 ul li.nav6 a span.over { background: #9dace9; }
- menu2 ul li.nav7 a span.over { background: #FFE66F; }
</style> <script type="text/javascript" src="http://ajax.googleapis.com/ajax/libs/jquery/1.3/jquery.min.js"></script> <script language="javascript"> $(document).ready(function() {
$("#menu2 li a").wrapInner( '' );
$("#menu2 li a").each(function() { $('' + $(this).text() + '' ).appendTo( this ); });
$("#menu2 li a").hover(function() { $(".out",this).stop().animate({'top':'45px'},200); $(".over",this).stop().animate({'top':'0px'},200);
}, function() { $(".out",this).stop().animate({'top':'0px'},200); $(".over",this).stop().animate({'top':'-45px'},200); });
});
</script> </head> <body>
</body> </html> <html>
Results,Discussion and Conclusion
</html>
Results
Self-assembly of iron nanoparticles under magnetic field
<html>
<img src="http://openwetware.org/images/9/92/HKBU2012COMPARENP.jpg" height="544" width="800"/>
</html>
We experimentally confirmed formation of stacked iron nanoparticle layers. Chaotic iron nanoparticles undergo self-assembly process to form unipolar and stacked pattern, with the help of magnetic field generated by conventional magnets. This pattern is expected to sustain and localize siRNA in a specific position for cell to uptake.
Cells under Confocal Microscope
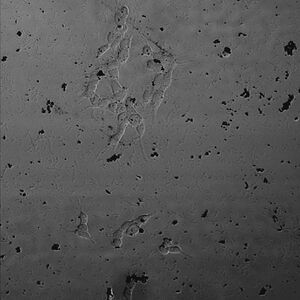
Cell viability after application of self-assembling iron nanoparticles is examined under Confocal Laser Scanning Microscope (CLSM). After iron nanoparticle treatment, SH-SY5Y cell with neuron cell characteristic can be observed under CLSM. The fully extended axons of individual cell reflect the prosperous growth of neuronal cells, which indicates the low toxicity of iron nanoparticles, fulfilling the most basic requirement for further biomedical application.
High-resolution images obtained from CLSM
After immunofluorescent treatment, the gene expression level in terms of signal intensity is detectable by direct imaging with CLSM. The higher the intensity, the higher the NR2B protein level, the lower the silencing effect of siRNA.
<html>
<img src="http://openwetware.org/images/7/76/Cell-2-R-P3_C002ee.jpg" height="200" width="200"/>
<img src="http://openwetware.org/images/6/63/Cell-2-R-P3_C001.jpg" height="200" width="200"/>
<img src="http://openwetware.org/images/4/47/HKBUCell-2-R-P3_%281%29.jpg" height="200" width="200"/>
| Cell(Bright View) | Cell(Dark View) | Cell(Combined Picture) |
</html>
<html>
Pictures above illustrate cell images under confocal microscope.
<img src="http://openwetware.org/images/e/e8/Cell.jpg" height="200" width="200"/> <img src="http://openwetware.org/images/0/08/Si.jpg" height="200" width="200"/> <img src="http://openwetware.org/images/e/e2/0.5-M%28-%29.jpg" height="200" width="200"/> <img src="http://openwetware.org/images/c/cd/0.5-M%28%2B%29.jpg" height="200" width="200"/>
| Cell | NR2B-specific siRNA | 0.5mg/mL NP+siRNA(without Magnetic Field) | 0.5mg/mL NP+siRNA(with Magnetic Field) |
</html>
From images above, we can see that compared to cell and siRNA control group, the fluorescent intensity of siRNA-nanoparticle complex with 0.5mg/ml concentration is significantly lower, which indicates a lower NR2B protein level and enhanced efficiency of siRNA.
SPSS Data Analysis Results
<html> <img src="http://openwetware.org/images/a/a4/HKBUcomparison_np.jpg" height="350" width="850"/> </html>
This graph is an illustration of images obtained from confocal microscope, showing fluorescent intensity of cells after nanoparticle-siRNA complexes treatment at different concentrations. The fluorescent signals are measured by Metamorph Software, and data analysis is conducted by SPSS to generate following graphs.
Comparison within control groups
<html> <img src="http://openwetware.org/images/2/22/HKBU2012RESULT1.jpg" height="440" width="650"/> </html>
This graph obtained from one-way anova data analysis illustrates that application of siRNA has the effect of reducing protein level of NR2B comparing with the cell control group (that is, no treatment has been done to the cells). However, the reduction level has not been significant yet. In addition, the magnetic field has almost no effect on siRNA only.
Comparison between different concentrations of iron nanoparticle with cell control
<html> <img src="http://openwetware.org/images/a/af/Hkbu2012result22.jpg" height="480" width="650"/> </html>
This graph obtained from one-way anova data analysis illustrates that application of nanoparticles carrying NR2B-specitic siRNA has significant effects of reducing protein level of NR2B, when compared with the cell control group. Among the groups with the magnetic field application, nanoparticles with 0.25mg/ml, 0.5mg/ml and 1mg/ml all have significant effect. Among the groups without the magnetic field application, only 0.5mg/ml has significant effect. All results listed here imply that the hypothesis of using nanoparticle to carry siRNA into cells in order to reduce the NR2B protein express has worked. Comparing within the group with magnetic field and the group without magnetic field, it is obvious that the group with magnetic field has a more strong effect on reducing the protein expression, which indicates that magnetic field could help nanopaticles to improve the carrying efficiency of siRNA into cells.
Comparison between different concentrations of iron nanoparticle with siRNA control
<html>
<img src="http://openwetware.org/images/d/d5/Hkbu2012result3.jpg" height="430" width="650"/>
</html>
This graph obtained from one-way anova data analysis illustrates the following points:
(1) Compared to siRNA control group, the decreasing fluorescent intensity in groups with nanoparticle-siRNA complexes demonstrate that nanoparticles carrying with siRNA has improved effect of reducing protein level of NR2B.
(2) Comparing between the group with magnetic field and the group without magnetic field, it is obvious that the group with magnetic field has stronger effect on reducing the protein expression, which indicates that magnetic field could facilitate iron nanoparticle-mediated siRNA delivery.
(3) Among groups with the magnetic field application, only 0.5mg/ml iron nanoparticle has a significant effect compared to control group. Among the groups without the magnetic field application, again, iron nanoparticle at 0.5mg/ml has significant efficiency as well. This result implies that iron nanoparticles with 0.5mg/ml concentration may be the optimum condition for stacking structure formation and siRNA transfection.
Discussions
Application of magnetic iron nanoparticle enhances NR2B-specific siRNA delivery
Based on our data analysis, the fluorescent intensity measured from images generated by CLSM in groups with iron-nanoparticles is lower than siRNA only control group, the results of which demonstrate the efficiency of iron nanoparticles in siRNA delivery. As siRNA is unstable and vulnerable to intracellular enzyme, low toxicity iron nanoparticles sustain gene effects in specific sites, prevent degradation of siRNA by enzymatic activities (proteases and nucleases), and hence, improve overall targeting efficiency of siRNA without reducing their therapeutic efficacy.
The application of magnetic field could further improve the efficiency of protein expression reduction
According to the results above, we found that the group with magnetic field has more effect on the protein expression reduction. An explanation could be that because of the unipolar stacking of the nanoparticles under the magnetic field, it could carry more siRNA during the process of carrying them into cells. Moreover, magnetic filed is able to enhance the localization of siRNA, direct the gene delivery, facilitate internalization of nanoparticles through endocytosis into cells, and hence, further improve gene delivery. However, the exact theory behind may require further investigation.
The concentration of 0.5mg/ml iron nanoparticle might be the most appropriate concentration for siRNA delivery
Among all those conditions we did, the concentration of 0.5mg/ml iron nanoparticle always have an excellent performance in the siRNA delivery. We conjecture that 0.5mg/ml iron nanoparticle might be the most appropriate concentration for siRNA delivery. It might due to that 0.25mg/ml nanoparticles are too diluted for the nanoparticles to carry enough siRNA into cells. However, the concentration of 0.75mg/ml or 1mg/ml might be too high that excessive nanoparticles tend to cluster together and trap siRNA within their complex layers. Since in our experiment, only four different concentrations are conducted, we could not conclude that 0.5mg/ml is the best condition for siRNA delivery. Further study should be conducted on a smaller concentration interval to narrow down to the best condition. However, we have a strong confidence that it should be around 0.5 mg/ml in general.
Conclusion
Our findings support the possible use of NR2B-specific siRNA in SH-SY5Y neuronal cell by magnetic nanoparticle based transfection for efficient silencing of NR2B gene. Results of laser scan microscope analysis demonstrate effectiveness of using self-assembly iron nanoparticles under magnetic fields in RNAi pathway. Through our in vitro experiments, we prove the feasibility and high efficiency of our approach by verifying the low cytotoxicity, self-assembly capacity of our iron nanoparticle, enhancement of gene silencing effects by applying of magnetic field on siRNA-nanoparticle complexes. In addition, an optimum concentration of iron nanoparticle at 0.5mg/ml is identified based on our present study.
Achievements
(1)We verify the self-assembly capacity of iron nanoparticles under magnetic field. And experimentally confirmed formation of stacked iron nanoparticle layers.
(2)We prove the cell viability after application of iron nanoparticle by our in vitro experiment.
(3)We demonstrate proof of the principle for the efficiency of self-assembled iron nanoparticles under magnetic field in nanoparticle-mediated gene expression.And an optimum concentration of nanoparticles for gene delivery in our experiment is identified.
Future study
We believe future study is necessary to complete our project and further prove our principles of utilizing iron nanoparticles under magnetic field in drug delivery.
-Other quantative means should be applied such as Western blot to generate more accurate results to further prove the efficiency of our approach.
-Cytotoxicity test should be applied to quantatively measure the biocompatibility and biotoxicity of iron nanoparticles used in our experiment.
-More concentration should be tested to further narrow down and minimize the range of optimum magnetic nanoparticle concentration in siRNA delivery.
In the future, in order to enhance the efficiency level of our approach, multiple techniques can be further investigated:
-Pre-position magnetic field generating agent into target cell to direct more efficient gene delivery
- Add functional group onto iron nanoparticle surface to extend the functionality and improve absorbing ability of nanoparticles, thus enhance siRNA-mediated target knockdown effects of nanoparticle.
<html> <a href="http://openwetware.org/index.php?title=Biomod/2012/HKBU/BU_Magician:Results&action=edit">edit</a> </html>