Banta:Gels

Banta Lab
Protein and Metabolic Engineering
| Home | Lab Members | Publications | Research Interests | Courses | Pictures | Positions Available |
Self-Assembling Protein Hydrogels for Bioelectrocatalysis
Self-assembly is an essential process for all forms of life. For example, proteins spontaneously fold into well-defined 3-dimensional structures, and cellular organelles form that spatially segregate diverse cellular processes. As engineers aim to create new devices and systems at ever decreasing size scales, self-assembly processes become increasingly attractive techniques.
We are interested in using protein self-assembly in bioelectrocatalytic applications including biofuel cells and biosensors. In these devices it is critical to have a high loading of enzymes on the electrode and to address transport issues including electrical communication with the electrode and substrate and product transport to and from the enzyme.
We have developed a technology where self-assembly domains are genetically appended to globular proteins and this enables the proteins to self-assemble into bifunctional hydrogels. We have performed this modification on over a dozen different proteins and in all cases the proteins retain their biological function (activity) while gaining the ability to form a biomaterial. We have demonstrated the use of these materials as electrode modifications for biofuel cells and biosensors. For example, we have created a hydrogel composed of three dehydrogenase enzymes that are able to form a metabolic pathway for the oxidation of methanol to carbon dioxide. This protein hydrogel was then used to make an enzyamtic biofuel cell. We are continuing to develop this approach to make catalytic biomaterials for additional applications.
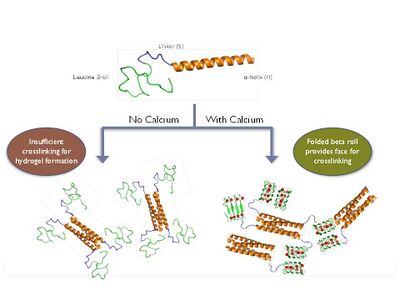
In our original protein hydrogels we used self-assembling alpha-helical appendages as cross-linking domains. We have now engineered the conformationally dynamic beta roll domain to serve as a calcium-dependent cross-linking motif. This allows for hydrogel formation to be controlled by calcium addition.
Related Publications
- Dooley K, Bulutoglu B, and Banta S. Doubling the cross-linking interface of a rationally designed beta roll peptide for calcium-dependent proteinaceous hydrogel formation. Biomacromolecules. 2014 Oct 13;15(10):3617-24. DOI:10.1021/bm500870a |
- Kim YH, Campbell E, Yu J, Minteer SD, and Banta S. Complete oxidation of methanol in biobattery devices using a hydrogel created from three modified dehydrogenases. Angew Chem Int Ed Engl. 2013 Jan 28;52(5):1437-40. DOI:10.1002/anie.201207423 |
- Dooley K, Kim YH, Lu HD, Tu R, and Banta S. Engineering of an environmentally responsive beta roll peptide for use as a calcium-dependent cross-linking domain for peptide hydrogel formation. Biomacromolecules. 2012 Jun 11;13(6):1758-64. DOI:10.1021/bm3002446 |
- Lu HD, Wheeldon IR, and Banta S. Catalytic biomaterials: engineering organophosphate hydrolase to form self-assembling enzymatic hydrogels. Protein Eng Des Sel. 2010 Jul;23(7):559-66. DOI:10.1093/protein/gzq026 |
- Banta S, Wheeldon IR, and Blenner M. Protein engineering in the development of functional hydrogels. Annu Rev Biomed Eng. 2010 Aug 15;12:167-86. DOI:10.1146/annurev-bioeng-070909-105334 |
- Wheeldon IR, Campbell E, and Banta S. A chimeric fusion protein engineered with disparate functionalities-enzymatic activity and self-assembly. J Mol Biol. 2009 Sep 11;392(1):129-42. DOI:10.1016/j.jmb.2009.06.075 |
- Wheeldon IR, Gallaway JW, Barton SC, and Banta S. Bioelectrocatalytic hydrogels from electron-conducting metallopolypeptides coassembled with bifunctional enzymatic building blocks. Proc Natl Acad Sci U S A. 2008 Oct 7;105(40):15275-80. DOI:10.1073/pnas.0805249105 |
- Gallaway J, Wheeldon I, Rincon R, Atanassov P, Banta S, and Barton SC. Oxygen-reducing enzyme cathodes produced from SLAC, a small laccase from Streptomyces coelicolor. Biosens Bioelectron. 2008 Mar 14;23(8):1229-35. DOI:10.1016/j.bios.2007.11.004 |
- Wheeldon IR, Barton SC, and Banta S. Bioactive proteinaceous hydrogels from designed bifunctional building blocks. Biomacromolecules. 2007 Oct;8(10):2990-4. DOI:10.1021/bm700858p |