BME100 s2016:Group8 W1030AM L5
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||||
OUR TEAM
LAB 5 WRITE-UPPCR Reaction ReportMembers of our team had extensive previous experience pipetting which led to little difficulty in setting up the reaction. The pre-lab reading was a good review of the technique but was aimed at people with less experience with the process. Because we knew the difference between the first and second stops our measurements were as accurate as possible, but there is always a possibility of error in a human-driven procedure. In a couple of the tubes there was a small amount of sample left. This could have been due to taking air into the pipettor instead of liquid or an accidental adjustment of the volume setting on the handle of the pipettor. In the final part of the set up, we came up with a good labeling scheme, but we had to relabel the sides of the tubes before putting them into the PCR machine in case the intense heat removed the ink from the lids. Fluorimeter ProcedureWeb camera set-up This experiment used an iPhone 6 camera without flash. None of the other settings could be changed manually. The phone was placed in the cradle so that the lens was level with and at a right angle to the slide and 8 cm from the front of the slide for each photograph and focused.
Placing Samples onto the Fluorimeter
Data Collection and AnalysisImages of High, Low, and Zero Calf Thymus DNA
Images of Our PCR Negative and Positive Controls
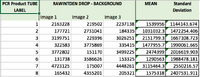
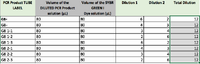
PCR Results: PCR concentrations solved
| |||||||