BME100 s2016:Group2 W1030AM L6
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||||
OUR COMPANY: LIBEDE TECH
Name of team's company: LIBEDE TECH Brand name of product: LibedePCRMachine LAB 6 WRITE-UPBayesian StatisticsOverview of the Original Diagnosis System For the analysis of patients’ DNA in order to make diagnoses, 16 groups from 16 different companies with each group containing six employees tested 32 patient samples using the process of PCR for the sake of division of labor. Therefore, each group tested two patient samples. Each group tested each patient sample three times by taking three drop images of each patient sample in order to prevent errors by attempting to increase the accuracy of the PCR results. One challenge that was faced was that the pictures were not of high quality, since they were taken with an iPhone 6 camera.
The probability of a patient getting a positive final test conclusion given a positive PCR reaction was close to 1.00 (100%). The probability of a patient getting a positive PCR reaction given a positive final test conclusion was close to 1.00 (100%). These results from calculation 1 mean that the individual PCR replicates were reliable for concluding whether or not a patient has the disease SNP.
1) Inaccurate pictures: Since the pictures were taken with an iPhone 6 camera, the colors of the drop images may not have been accurate. Also, the dim lighting cause the pictures to be a little blurry. This error could be minimized by using a professional camera. While all groups used ImageJ to analyze the drop images from the fluorimeter, some used webcam cameras to take the pictures while others used phone cameras. The use of different cameras probably contributed to the error in the experiment, because the type of camera that was used should have been controlled. 2) Small number of trials: Each patient sample was tested only three times. In order to increase the possible accuracy of the results, more trials could be performed in the future. 3) Concentration of DNA: If the concentration of DNA was very low, it is possible that tests performed on the DNA would not be accurate. Intro to Computer-Aided DesignTinkerCAD Our Design
Feature 1: ConsumablesThe LibedeTech consumables kit will include:
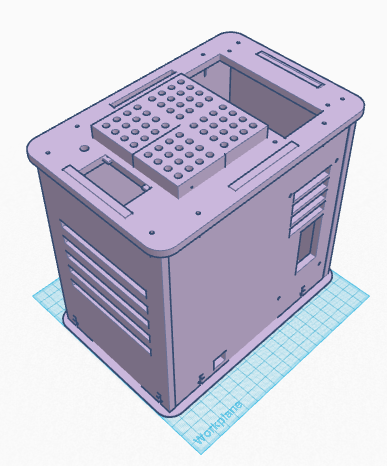
The packaging of the consumables will contain all of the needed supplies packaged separately, there will also be a full list of instructions for when the subject is setting up the PCR machine. Each of the separately packaged supplies will also have a label, addressing the name of the supply and what it is used for. Among other things, there will be a disposable bag that can be used when disposing the equipment, especially the pipette tips. Pictures and diagrams will also be provided for a visual view of the PCR machine after it is set up. The PCR tubes rack will also be in the kit along with the necessary mixtures to perform the experiment. The test tube rack will be larger compared to others to minimize the previously stated problem with not having enough sample space. Feature 2: Hardware - PCR Machine & FluorimeterThe PCR Machine that was used for the experiment was very limiting in that it only allowed 16 slots for PCR samples to be placed into the machine. For this reason, the new and improved LibedePCRMachine was upgraded to have 64 slots which makes the machine more efficient.
| |||||||