Austindias Week 7
Purpose
The objective for this week is to reach a biological understanding of the modeling we completed in the previous week. Analysis of the data will be used to develop ideas on how these genes are interacting with each other. Moving forward, the input excel workbook will be modified and the modeling results will be analyzed to observe the result of this change on the modeling results.
Methods
- Observe Dynamical Systems Modeling Results from MATLAB
- Observe output excel workbook from MATLAB
- Observe log 2 fold changes in comparison to optimized fold changes given from the model on GRNsight for each strain
- Analyze the results including the fit of the model, comparison of gene interactions in different yeast strain types, expression and repression of different genes and the transcription factors that are regulating them
- Develop a hypothesis for a proposed change to the input excel workbook for MATLAB. Explain why this modification was selected and the expected results. After running the tweaked model, the difference between the original model and changed model can be observed to understand exactly how this change effected the model and gene expression.
- Made a modification to the input modeling workbook to include all timepoints up to 120 minutes.
- Analyzed results from all data and modeling to construct a presentation with clear conclusions about cold shock genes.
Analyzing Results of First Model Run (t0-60)
What is the overall least squares error (LSE) for your model?
0.689996224
LSE:minLSE ratio
1.3316
Genes that appear to be modeled well according to Dynamical Systems Modeling Results:
- MSN4
- CRZ1
- GIS1
- ADR1
- MIG1
- MGA2
- SKO1
- RAP1
- PHO2
- YHP1
Genes that do not appear to be modeled well according to Dynamical Systems Modeling Results:
- HAP4
- GLN3
- SUT1
Comparing the actual data for each strain, with the simulated data from the same strain. If the model fits the data well, the color heatmap superimposed on the node will match top and bottom. If the fit is less good, the colors will not match.
wt_log2_expression versus wt_log2_optimized_expression
Genes that are modeled well:
- ADR1
- GLN3
- MGA2
- MIG1
- MSN4
- PHO2
- SKO1
Genes that are not modeled well:
- CRZ1
- HAP4
- SUT1
- YHP1
dGLN3_log2_expression versus dGLN3_log2_optimized_expression
Genes that are modeled well:
- GIS1
- MGA2
- MIG1
- MSN4
- PHO2
- SKO1
- SUT1
Genes that are not modeled well:
- ADR1
- CRZ1
- GLN3
- HAP4
- RAP1
- YHP1
dHAP4_log2_expression versus dHAP4_log2_optimized_expression
Genes that are modeled well:
- ADR1
- CRZ1
- GIS1
- GLN3
- HAP4
- MGA2
- MIG1
- PHO2
- RAP1
- SKO1
- SUT1
Genes that are not modeled well:
- MSN54
- MSN4
- YHP1
Bar Charts are included in this file for b_threshold and parameters. 13 genes_Bar_charts_ouputdata_AD
Analyzing Results of First Model Run (t0-120)
What is the overall least squares error (LSE) for your model?
0.863437413405097
LSE:minLSE ratio
1.436
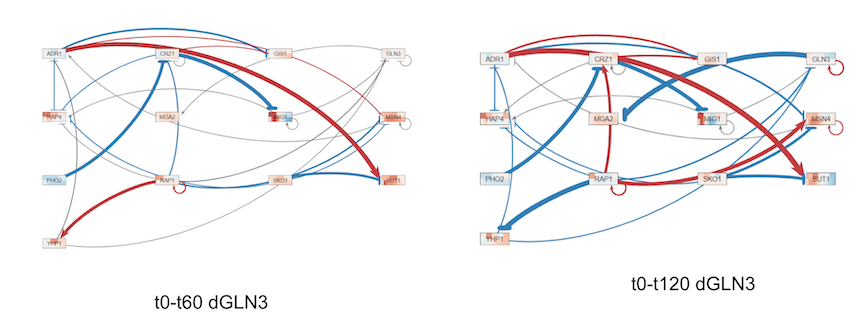
Graphs comparing optimized values for t0-t60 versus t0-t120
Adding t90-t120 timepoints did not greatly improve the model over initial run. Discrepancies between log fold change expression and optimized data were still present.
- RAP1 changes from influencing YHP1 to be expressed to influencing YHP1 to be repressed when the modification was made.
- RAP 1 changes from influencing CRZ1 to be repressed to influencing CRZ1 to be expressed when the modifications was made.
- GLN3 shows greater influence on target genes in modified model.
Conclusions from Part 1
YHP1 is a gene that is not modeled well in any of the strains. This result is somewhat surprising because YHP1 in the gene network is only acted on by one transcription factor in RAP1. However, YHP1 was a weak influence on ADR1 and GLN3. Besides in the strain dHAP4, HAP4 was not modeled well. HAP4 is a gene that is very involved in the gene network and is repressed by several different genes. The model appeared to be the least accurate for the dGLN3 yeast strain. MIG1 displays the most dramatic change in expression due to cold shock. In each strain it shows a strong expression earlier in the cold shock treatment, followed by a repression later on. This gene is controlled by the transcriptional factor CRZ1. However, CRZ1 within this experiment appeared to only have a repressing ability on MIG1, so it is unknown which factor is causing MIG1 to show increased expression early in cold shock. MIG1 was found to have the ability to act on itself, but with minimal weight that does not seem to match the magnitude of the log 2 fold change. MIG1 was one of the few genes that displayed a visible change in expression. For most of the genes in this network, there is no log 2 fold change greater than +2 or less than -2 within the first 60 minutes of the experiment. Initially, this profile of genes was selected for further study because of their increased expression around 90 minutes. However, this model only used data up to 60 minutes. As a result of this, I would be highly interested to see how extending the time points to 120 minutes would effect the model and lead to a more accurate representation of gene expression in this network.
Conclusions for Part 2
Extending the data to include timepoints up to t190 helped to reveal the complete story regarding this gene network and their expression both during cold shock and recovery periods. The inclusion of t90 and t120 drastically changed optimized results for threshold values, network weights, and production rates (Refer to graphs file in results section). However, the LSE:minLSE ratio was not improved by this modification. Within the network for the dGLN3 yeast strain, initially GLN3 had little affect on any of its target genes. Once the modification was made, GLN3 exhibited strong repression effect on MGA2. This network weight is important to consider because this strain did not contain dGLN3, so you would not expect this gene to have such a strong influence on MGA2. Between t0-t60 RAP1 has a somewhat strong influence on expression of YHP1 and repression on CRZ1 in the dGLN3 strain. However, both of these relationships are reversed when including all timepoints, suggesting a significant change in behavior of YHP1 and CRZ1 during the recovery period to allow the cell to return to its homeostatic condition.
Combinatorial Control of MIG1 & MIG2 are Likely Regulators of the Early Cold Response in Yeast (S. cerevisiae)
- PowerPoint Presentation
Acknowledgements
- I would like to mention the text and in class interactions Leanne Kuwahara for answering questions on the assignment, as well as comparing results and discussing where we should head next in terms of data analysis.
- Dr.Dahlquist was helpful in explaining the biological meaning of GRNsight and how we can analyze our dating using this platform.
- Dr.Fitzpatrick provided insight into what the model was actually doing and how our model relates to the raw data. He also explained some of the inferences we can make from the data we collected.
Except for what is noted above, this individual journal entry was completed by me and not copied from another source
Austindias (talk) 21:32, 20 March 2019 (PDT)
References
Dahlquist, K. and Fitzpatrick, B. (2019). BIOL388/S19:Week 7. [online] openwetware.org. Available at:Week 7 Assignment Page [Accessed 6 March. 2019].
GRNsight. (n.d.). Retrieved from http://dondi.github.io/GRNsight/
Return to Homepage Austin Dias