Acoustofluidic Mixing using Piezoelectronics - Jamar Hawkins
Introduction
Piezoelectric transducers are used in everyday devices to transfer mechanical stress into electrical signals. As the piezoelectric effect is an intrinsic and reversible capability of certain materials, the transducers are usually simple devices made from just two layers, a piezo ceramic and metal substrate, and operate extremely fast. The simple design of piezo transducers allows them to be small, inexpensive and easy to use. Many different technologies from pressure sensors to voice recognition devices use these transducers as they are easy to implement in even the thinnest electronic devices. Common devices using them include cell phones and medical ultrasound machines.
Piezo Transducers in Scientific Literature
A very attractive aspect of acoustofluidic actuation in microfluidic devices is that it does not add any biocompatibility or contamination concerns as piezo transducers are capable of actuating from outside the microfluidic channel. This ‘contact free’ actuation can be achieved when a piezo transducer is bonded to a material such as poly(dimethylsiloxane) (PDMS) and creates an acoustic wave that travels along the surface of the medium. This type of wave is known as a surface acoustic wave (SAW) and is usually characterized as a high frequency (up to 450 MHz) and variable amplitude wave, which can very easily dissipate into small volumes of fluid.[1] This capability has been leveraged in acoustofluidics for cell separation, acoustic pumping and microfluidic mixing.[1-8] To help highlight why academics find this interesting enough to publish in their top microfluidics journals such as Lab on a Chip, we will discuss the limitations typically introduced at microfluidic scales. Though performing chemical reactions in microfluidic devices is an attractive possibility due to the cost efficiency of using low volumes of reactants, the small length scales employed have the consequence of flow parameters dominated by viscosity and diffusion-dominated mass transport. This creates the advantage of easily forming steady chemical gradients but is disadvantageous in applications requiring mixing. [5] Clearly, this presents an issue in performing chemical reactions requiring mixing of multiple reagents in microfluidic devices. Generating acoustic waves in a microfluidic channel can introduce turbulence and vortices, especially when coupled with deformable polymer structures within the channel. This facilitates a degree of mixing within microfluidic channels that could not be attained in standard microfluidic devices.
Acoustofluidic-Actuated Polymer Cilia
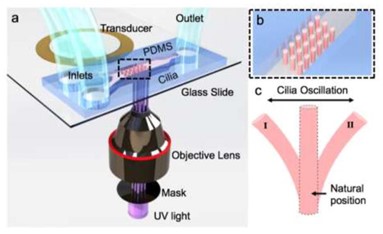
The first acoustofluidic design we will discuss is one created by Orbay et al. in 2018. This design uses a standard piezo transducer to oscillate high aspect ratio poly(ethylene glycol) diacrylate (PEGDA) pillars in a microfluidic channel (Figure 2). [9] The piezo transducer is bonded to the microfluidic chip via a thin layer of epoxy and operated at its resonance frequency (~4.6 kHz) for maximum pillar deflection. The authors suggest that the direction of the pillar oscillation is determined by the wave pattern on the glass substrate. The oscillations of the pillars allowed for localized mixing in their microfluidic channel, which the authors used DI water and fluorescein dye solutions to demonstrate and a dimensionless value called the sperm number to quantify. This device presents and interesting way to allow for rapid fluid mixing with spatial control. The external method of actuation engenders a great deal of design freedom regarding the material and can certainly allow for biocompatible materials which operate in sterile conditions. Furthermore, the user can still simply leave the transducer turned off to achieve a gradient if needed. However, it should be noted that the authors’ claims of low-cost and accessibility remain unclear as the waveform generators and signal amplifiers needed to operate the device at a sufficient signal are several thousands of dollars. Overall, this device is an impressive example of the simplicity and effectiveness of acoustofluidic mixing.
Acoustofluidic Mixing Using Cavitation Bubbles
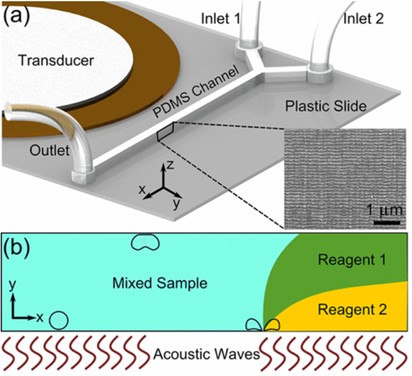
Another interesting acoustofluidic mixing device has been reported by Ozcelik et al. in 2014. Their setup utilizes rough PDMS microchannel side walls to initiate and cavitate bubbles under the influence of acoustic waves. [10] Like the last device we discussed, the piezo transducer is bonded near, but outside, the PDMS channel using epoxy. Meaning we again see SAWs used for actuation. The rough surface of the PDMS traps small amounts of air inside when injected with liquid. These small amounts of air form cavitation nuclei when the acoustic field is applied to the surrounding liquid. When the transducer is functioning, the resulting acoustic waves undergo compression and expansion cycles. During the expansion cycle the diffusion boundary layer of the bubbles gets thinner while the surface area of the bubble gets larger. This results in gas being transferred from the surrounding medium to the bubble. The cavitation of these bubbles is very fast, generating both jetting and counter rotating vortices. These vortices break the laminar flow and allow for fast homogenous mixing. Impressively, the authors demonstrate this device can mix two viscous solutions of poly(ethylene glycol) (77.3 mPa * s) within 100 ms. Again, we see an acoustofluidic device that leverages fine structures within a microchannel to achieve microfluidic mixing. The ability of this device to quickly mix such viscous solutions is impressive and has potential for fast mixing of polymer solutions as it can achieve turbulent flow regimes in very small length scales. However, we again see the same issue with the high cost of equipment required for the operation of this device. Specifically, the requirement of a waveform generator and signal amplifier. This certainly interferes with the potential of acoustofluidics for translation outside of the research setting.
Conclusion
Acoustofluidics is an impressive field with the capabilities to accommodate even the most sensitive chemical and biomedical applications. It also has great potential for mediating some of the inherent issues at microfluidic scales. The two examples we saw in this chapter discuss how we can use acoustofluidics in combination with lithographic patterning to achieve spatial and temporal control of mixing in microfluidic devices. Although the setup cost of operating such devices does present a challenge for widespread implementation outside of research settings, it certainly has potential to advance microfluidics as field as well.
References
[1] Z.G. Kharaji, M. Bayareh, V. Kalantar, A review on acoustic field-driven micromixers, Int. J. Chem. React. Eng. 19 (2021) 553–569., doi:10.3390/mi8090274
[2] N.H. Le, N.H. Deng, C. Devendran, N. Akhtar, X. Ma, C.H. Pouton, H.K. Chan, A. Neild, T. Alan, Ultrafast star-shaped acoustic micromixer for high throughput nanoparticle synthesis, Lab Chip 20 (2020) 582–591. DOI https://doi.org/10.1039/C9LC01174A
[3] Y. Lin, C. Gao, Y. Gao, M. Wu, A.A. Yazdi, J. Xu, Acoustofluidic micromixer on labon-a-foil devices, Sens. Actuators B: Chem. 287 (2019) 312–319. https://hdl.handle.net/10027/23436
[4] S. Zhao, P.H. Huang, H. Zhang, J. Rich, H. Bachman, J. Ye, W. Zhang, C. Chen, Z. Xie, Z. Tian, P. Kang, H. Fu, T.J. Huang, Fabrication of tunable, high-molecular-weight polymeric nanoparticles via ultrafast acoustofluidic micromixing, Lab Chip 21 (2021) 2453–2463. DOI https://doi.org/10.1039/D1LC00265A
[5] E. Lim, L. Lee, L. Y. Yeo, Y. M. Hung and M. K. Tan, "Acoustically Driven Micromixing: Effect of Transducer Geometry," in IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, vol. 66, no. 8, pp. 1387-1394, Aug. 2019, doi: 10.1109/TUFFC.2019.2920683.
[6] Schmid, L., Weitz, D. A. & Franke, T. Sorting drops and cells with acoustics:acoustic microfluidic fluorescence-activated cell sorter. Lab Chip 14, 3710–3718 (2014). DOI https://doi.org/10.1039/C4LC00588K
[7] Sethu, P., Sin, A. & Toner, M. Microfluidic diffusive filter for apheresis (leukapheresis). Lab Chip 6, 83–89 (2006). DOI https://doi.org/10.1039/B512049G
[8] Link, D. R. et al. Electric control of droplets in microfluidic devices.Angew.Chem. Int. Ed 45, 2556–2506 (2006) doi: 10.1002/anie.200503540.
[9]Orbay S, Ozcelik A, Bachman H, Huang TJ. Acoustic Actuation of in situ Fabricated Artificial Cilia. J Micromech Microeng. 2018;28(2):025012. doi:10.1088/1361-6439/aaa0ae
[10] Ozcelik, A. et al. An Acoustofluidic Micromixer via Bubble Inception and Cavitation from Microchannel Sidewalls. Anal Chem 86, 5083-5088, doi:10.1021/ac5007798 (2014).
