20.109(F11): Mod 1 Day 3 Agarose gel electrophoresis
Agarose gel electrophoresis
Introduction
Electrophoresis is a technique that separates large molecules by size using an applied electrical field and a sieving matrix. DNA, RNA and proteins are the molecules most often studied with this technique; agarose and acrylamide gels are the two most common sieves. The molecules to be separated enter the matrix through a well at one end and are pulled through the matrix when a current is applied across it. The larger molecules get entwined in the matrix and retarded; the smaller molecules wind through the matrix more easily and travel further from the well. Molecules of the same size and charge migrate the same distance from the well and collect into a band.

DNA and RNA are negatively charged molecules due to their phosphate backbone, and they naturally travel toward the positive charge at the far end of the gel. They are typically examined with agarose gels. Proteins are composed of amino acids that can be positively, negatively or uncharged. To give proteins a uniformly negative charge, they are coated with a detergent, SDS, prior to running them on a gel. Protein samples are also boiled to remove any secondary structure that might make two molecules of the same size migrate differently. Polyacrylamide is the matrix commonly used to separate proteins. These gels are typically run vertically while agarose gels are run horizontally but gravity has nothing to do with the separation.
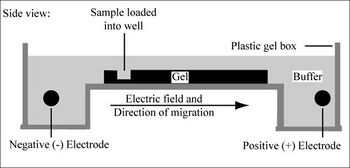
Today you will separate DNA fragments using an agarose matrix. Agarose is a polymer that comes from seaweed and if you’ve ever made Jell-O™, then you already have all the skills for pouring an agarose gel. To prepare these gels, agarose and buffer are microwaved until the agarose is melted. The molten agar is then poured into a horizontal casting tray, and a comb is added. Once the agar has solidified, the comb is removed, leaving wells into which the DNA sample can be loaded.
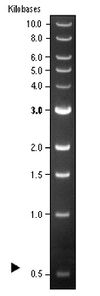
The distance a DNA fragment travels is inversely proportional to its length. Over time fragments of similar length accumulate into “bands” in the gel. Higher concentrations of agarose can be used to resolve smaller DNA fragments. The figure below (far right) shows the same DNA fragments resolved with three agarose concentrations. The 1000 base pair fragment is indicated in each.
- Agarose gel electrophoresis
-
Scanning EM image of agarose polymer
-
Agarose gel data analysis
-
Agarose gel raw data
Ethidium Bromide is a fluorescent dye that is commonly added to agarose gels. This dye intercalates between the bases of DNA, allowing DNA fragments to be located in the gel under UV light and photographed. The intensity of the band reflects the concentration of molecules that size, although there are upper and lower limits to the sensitivity of dyes. Because of its interaction with DNA, ethidium bromide is a powerful mutagen and will interact with the DNA in your body just as it does with any DNA on a gel. You should always handle all gels and gel equipment with nitrile gloves. Agarose gels with Ethidium Bromide must be disposed of as hazardous waste.
Today you will run your digested plasmid backbone and the digested PCR product on an agarose gel. You will then cut the relevant bands of DNA out of your gel and proceed to purify them from the agarose. Next time you will mix them in a ligation reaction.
Protocol
Part 1: Running your gel
You will use a 1% agarose gel (prepared by the teaching faculty), running nine samples as well as a reference lane of molecular weight markers (also called a DNA ladder).
- Add 20 μL of sterile water to the 5 μL PCR aliquots (not the PCR double digest) you stored after Mod1 Day2.
- Add 2.5 μL loading dye to the PCR aliquots, as well as to the pCX-NNX digested by XbaI and/or EcoRI, and the PCR product digested by XbaI/EcoRI.
- Loading dye contains xylene cyanol as a tracking dye to follow the progress of the electrophoresis (so you don’t run the smallest fragments off the end of your gel!) as well as glycerol to help the samples sink into the well.
- Flick the eppendorf tubes to mix the contents, then quick spin them in the microfuge to bring the contents of the tubes to the bottom.
- Load the gel in the order shown in the table below.
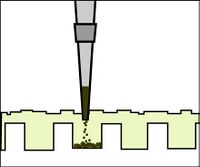
- To load your samples, draw the volume listed on the table below into the tip of your P200. Lower the tip below the surface of the buffer and directly over the well. You risk puncturing the bottom of the well if you lower the tip too far into the well itself (puncturing well = bad!). Expel your sample into the well. Do not release the pipet plunger until after you have removed the tip from the gel box (or you'll draw your sample back into the tip!).
- Once all the samples have been loaded, attach the gel box to the power supply and run the gel at 125 V for no more than 45 minutes.
- You will be shown how to photograph your gel and excise the relevant bands of DNA.
| Lane | Sample | Volume to load |
|---|---|---|
| 1^ | Uncut pCX-NNX^ | 10 μL^ |
| 2 | pCX-NNX XbaI | 5 μL |
| 3 | pCX-NNX EcoRI | 5 μL |
| 4 | pCX-NNX XbaI + EcoRI | 25 μL |
| 5 | 1Kb DNA Ladder | 10 μL |
| 6 | PCR Product XbaI + EcoRI | 25 μL |
| 7 | PCR Product Uncut | 25 μL |
| 8 | PCR no-template-control | 25 μL |
^Available from the teaching faculty
Part 2: Communication of Biological Engineering
Once the gels are running, we will have an presentation from our writing faculty about communication of information through figures and legends. If you have loaded your gel and have time before they arrive (and your lab notebook is up to date!), you can look at the major communication assignments we have on tap for this term.
Part 3: Isolating/Purifying DNA
To purify your DNA from the agarose, you will use another kit sold by Qiagen. Again the reagents have uninformative names and their contents are proprietary. Like the PCR Clean-up kit, the Agarose Purification kit requires binding the DNA to a spin-column with a silica-gel membrane, washing away salts and eluting the DNA from the membrane.
- Estimate the volume of your gel slices by weighing them.
- Add 3 volumes of QG for every 1 volume of agarose. (The maximum advised volume is 550 ul.)
- Incubate in the 50°C water bath for 10 minutes, until the agarose is completely dissolved. Every few minutes, you can remove your tubes from the 50°C heat to flick the contents. This will help dissolve the agarose.
- Add 125 μL of isopropanol to each eppendorf tube.
- Get two QIAquick columns and two collection tubes from the teaching faculty. Label the spin-column (not the collection tubes!) either “bkb” or “frag” then pipet the appropriate dissolved agarose mixture to the top. Microfuge the column in the collection tube for 60 seconds. The maximum capacity of the QIAquick columns is 800 uL! If you have more than 800 uL in your mixture, you will need to repeat this step.
- Discard the flow-through in the sink and replace the spin-columns in their collection tubes. Add 750 ul of PE to the top of the column and spin as before.
- Discard the flow-through in the sink and replace the spin-columns in their collection tubes. Add nothing to the top but spin for 60 seconds more to dry the membrane.
- Trim the cap off two new eppendorf tubes and label the sides with your team color, the date, and either “bkb” or “frag.” Place the spin-column in the correct trimmed eppendorf tube and add 30 ul of EB to the center of the membrane.
- Allow the columns to sit at room temperature for one minute and then spin as before. The material that collects in the bottom of the eppendorf tubes is your purified plasmid backbone or insert, ready to be ligated.
Part 4: Evaluate recovery
Ligations generally work best when there is a 1:4 ratio of backbone to insert. You will ligate your fragments next time, but before you do, run a small amount out of the purified products on a gel. This will allow you
- to check that you purified your DNA from the agarose
- to assess the quality of the DNA (that it’s not degraded or contaminated)
- to adjust the amount of backbone and fragment in your ligations for an ~1:4 ratio.
1. Move 5 μL of each sample into an eppendorf tube labeled “purif bkb” or “purif frag.” Add 15 μL sterile water to each tube and 2 ul loading dye. Use a colored label to identify your team color and give the samples to the TA. The TA will run a 1% agarose gel for you and the result will be posted. You should examine the image before coming to lab next time (see “for next time” section).
2. Freeze the remainder of your purified DNAs at –20°C.
DONE!
For next time
- Take the log10 of the length of each molecular weight marker you can identify on your agarose gel photograph. Graph the log10 of their length on the y-axis versus the distance they migrated from the well on the x-axis, measured in mm using a ruler and the picture of your agarose gel. An example of such a graph is found in the introduction to today’s experiment. Use the equation of the line from your graph to determine the size of your PCR product and the pCX-NNX backbone. How do these compare with the predicted sizes?
- Prepare a properly labeled figure of your pCX-NNX/PCR product agarose gel, and write a legend for it. This will become a figure in your powerpoint summary.
- Once it is posted, examine the gel with your purified backbone and insert. Crop the gel to isolate the lanes you are considering as well as the marker lanes. Label the lanes and then determine the relative volumes you would use to give bands of *equal* intensity. For example if the backbone looks 10X brighter than the PCR product, you would use 10 μL of insert for every 1 μL of backbone.
Reagents list
- Loading Dye
- 0.25% xylene cyanol
- 30% glycerol
- RNase
- 1% agarose gel in 1X TAE
- 1X TAE
- 40 mM Tris
- 20 mM Acetic Acid
- 1 mM EDTA, pH 8.3