20.109(F07): Transmission electron microscopy
Introduction

Much of biology examines natural components that are too small to see. Imaging technology took a gigantic step forward in the 1680s when Anton van Leeuwenhoek ground a microscope lens sufficiently fine to see a living cell (a bacteria he had scraped from his teeth!). His microscope had one lens and the image he saw was approximately 250 times its natural size (250X magnification). Compound microscopes, like the ones we have in lab, use a second lens to magnify the image from the first and can increase the total magnification up to 1000X. However, no matter how fine its lens, a light microscope cannot distinguish objects closer than 200 nm. The resolution of light microscopes is limited by both the wavelength of white light (300-700 nm) and the scattering of light by the object it strikes. For better resolution, great lenses must be combined with shorter wavelengths, such as those followed by electrons or lasers, and better ways of focusing the beam such as forcing it to travel through a vacuum or an oil. Linking the microscope to a computer with digital image processing can also enhance its images. The sample itself can also be stained or fluorescently tagged to improve detection of its features.
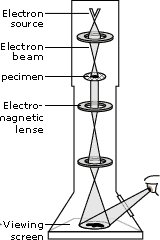
The Transmission Electron Microscope (TEM) achieves its remarkable resolution by “illuminating” samples using an electron beam in a vacuum rather than using a conventional light source in air. Since the electron beam passes through the sample that is being examined, the sample must be sufficiently thin and sufficiently sturdy to be hit by electrons in a vacuum. It’s important to remember that many biological materials are damaged or destroyed by the incoming electrons and that the TEM can image only the species that survive this harsh treatment. The denser parts of the sample will absorb or scatter some of the electron beam, and it’s the scattered electrons or those that pass through the sample that are focused using an electromagnetic lens. This “electron shadow” then strikes a fluorescent screen, giving rise to an image that varies in darkness according to the sample's density. For samples that are amenable to TEM, this form of examination can allow observation of angstrom-sized objects and of cellular details down to near atomic levels.
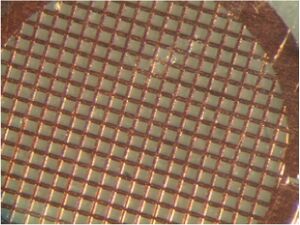
Samples are applied to a wafer-thin "grid" before being loaded into the TEM and placed under vacuum. The grid can be made of many kinds of materials. All have lines of a conductive metal, in our case copper, that disperse the electron beam and thereby help keep the sample from being blown to bits by the energy in the beam. A carbon mesh is strung between the metal lines. Once a sample has been applied to the grid, it's only the portions that come to rest on the carbon mesh can be visualized, along with any imperfections in the carbon mesh itself.
As you observe you samples today, you should keep in mind the artifacts and the restrictions that are intrinsic to this form of observation. For example, a dispersed phage nanowire solution may appear clumped or fragmented once the vacuum and electrons of the TEM have been applied. Nonetheless, this method is a powerful and interesting way to examine your materials and even some qualitative observations of the material you have built should give you confidence that the experiment is progressing as described.
Protocols
Part 1: TEM sample preparation
- Retrieve your dialyzed sample from the beaker of water and very carefully remove the top clip. The tubing will be slippery and you will be very sad if it slips out of your hand and spills.
- Use your P1000 to sip the sample out of the tubing, squeezing the liquid that's in the tubing toward the tip of the P1000. Place your iridium-coated phage (= nanowires) in an eppendorf tube. You will use only a small aliquot of this solution today but be sure to give the rest of your nanowire sample to the teaching faculty before you leave.
- Place 15 ul of your nanowire solution on the shiny, bright side of the TEM grid that you have balanced in the specialized tweezers. Treat the grid with care and use the tweezers only on the edge to minimize damaging the delicate mesh.

TEM grid balanced in tweezers - Allow the nanowires to settle onto the grid undisturbed for 30'.
- Remove the droplet from the grid with your P200 set to 50 ul.
- Wash the grid by adding 15 uL of sterile H2O onto the grid. Immediately remove the water.
- Dry the grid by very gently touching the edge of the grid to a piece of blotting paper in a petri dish. Place the grid into the TEM grid holder to transport to the TEM facility.
- To get there you should turn right as you leave our teaching lab and walk to the end of the hall to the "EXIT." Take the stairs down to the first floor and on your right you'll see a door to the core TEM facility that is run by the Center for Materials Science and Engineering. A member of the teaching faculty will be in the facility to examine your samples with you.
Part 2: TEM
At the TEM facility your sample will be placed in a holder to load into the microscope. It will take a few minutes for the machine to reach sufficient vacuum and then the teaching faculty will scan the grid for some interesting features to focus on and photograph. You should take notes about what you see so you can reconstruct the information to complete your FNT assignment. You need not return to the lab when you are done unless you've left something there.
DONE!
For next time
- You should prepare a figure of the TEM image you like best. This figure should include a legend like one you would find in a peer-reviewed journal article. To access your image go to this site. On the upper right side, you may see "If you need the EM Lab File Server, click HERE". Click there and you should see a lot of directories. Find "Natalie" and then identify your image from the others in the class to download.
- Update the class wiki page that catalogs the experimental variations. Be sure to comment on the TEM results you saw so there is a record of how successful each variation was.
- Continue to develop your research proposal and its presentation. Next time you will have a chance to work out more details with your lab partner and then share your idea with another lab group. Collect enough material to make good use of this upcoming time.
