Wet Lab Work - Envz and Tar - Glu
Ran PCR - June 1, 2012 (12-2)
Goal:
PCR of Tar and Envz with alanine insert.
Results:
Ran the PCR using primers 193 and 194 and saved the results: (12-2)
Primer 194 has an Alanine insert.
Ran PCR - June 1, 2012 (12-3)
Goal:
PCR of Tar and Envz with aspartate insert.
Results:
Ran the PCR using primers 193 and 195 and saved the results: (12-3)
Primer 195 has an Aspartate insert.
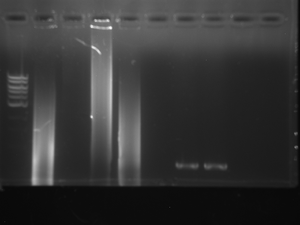
Gel of previous PCR - June 5, 2012 (13-1)
DR JA
Goal:
Get a band size: 200-300 base pairs
Lane order: Ladder, control, 12-2, 12-3.
Results:
Perfect Bands for both.
DNA Purification - June 5, 2012 (13-2)
Goal:
Purify PCR product with Alanine insert(12-2 PCR product tube).
Results:
Purified PCR Product (13-2).
DNA Purification - June 5, 2012 (13-3)
Goal:
Purify PCR product with Aspartate insert (12-3 PCR product tube).
Results:
Purified PCR Product (13-3).
Ran PCR - June 8, 2012 (15-3)
Goal:
Second step of chimeric PCR of Tar and Envz (Taz) with Alanine insert.
Results:
Ran the PCR using 13-2 as forward primer and 196 EnvZ reverse primer and saved the results: (15-3)
The PCR product used as a primer, 13-2, has an Alanine insert. JA
Ran PCR - June 8, 2012 (15-4)
Goal:
Second step of chimeric PCR of Tar and Envz (Taz) with Aspartate insert.
Results:
Ran the PCR using 13-3 as forward primer and 196 EnvZ reverse primer and saved the results: (15-4)
The PCR product used as a primer, 13-3, has an Aspartate insert. JA
WE SHOULD HAVE PURIFIED THE PCR PRODUCT (BUT WE DIDN'T), BUT IT WAS NO LOSS BECAUSE THE LOW-MELT GEL DIDN'T WORK (SEE BELOW).
Restriction Digest of Insert 13-2 and 13-3 - June 18, 2012 (15-7)
Reagents and Protocol:
- ddH2O (14 μL)
- PCR product (30 μL)
- 10x NEB Buffer #4 (5μL)
- 100x BSA (0.5μL)
- SacI (1.5μL)
- XbaI (1.5μL)
1. Mix all together and put in water bath for at least 2 hours or overnight at 37°C.
Tubes with digested inserts labeled "15-7 Asp" and "15-7 Ala."
Low- Melt Gel for Purification of the Sticky-ended Insert - June 19, 2012 (16-0)
Goal:
Purify the digests of 15-7 Asp and 15-7 Ala.
Results:
We ran 15-7 Asp and 15-7 Ala on the gel to purify it, but the gel did not work because the gel was too thin. We will restart the process by re-amplifying the Tar-EnvZ chimeric protein using 13-2 and 13-3 as our DNA templates. BR JM
Re-amplification of Chimeric Tar-EnvZ - June 19, 2012 (16-1)
We used 13-2 as a template in the first reaction, and 13-3 as a template in the second reaction, with BI193 and BI196 as our primers for both reactions. We used the following protocol:
Phusion PCR Reagents (50 μL total):
- ddH2O (35μL)
- 5x Phusion Buffer (10μL)
- 10 mM dNTPs (1.5μL)
- Forward Primer BI193 (1μL)
- Reverse Primer BI196 (1μL)
- template DNA (1μL) - either 13-2 or 13-3
- Phusion Polymerase (.5μL)
16-1 Ala (13-2 used as template)
16-1 Asp (13-3 used as template)
BR JM
Purification of Chimeric Tar-EnvZ - June 21, 2012 (16-2)
Goal:
Purify 16-1, the Chimeric Tar-EnvZ protein with Ala and Asp inserts.
Results:
We purified 16-1 Asp and 16-1 Ala using the protocol found in the Sigma-Aldrich PCR Clean-Up Kit.
-Insert mini spin column into collection tube and add 0.5 ml of column prep solution.
-Centrifuge for 30 sec to 1 minute.
-Add 5 volumes of binding solution to 1 volume of PCR mix and transfer solution to the binding column.
-Centrifuge for one minute.
-Discard the eluate and replace the binding column in the collection tube.
-Apply 0.5 ml of wash solution and centrifuge for 1 minute.
-Replace the column into the collection tube and centrifuge for an additional 2 minutes without adding any solution, discard the eluate.
-Place the column into a new collection tube and add 50 μl of Elution Solution. Let the tube sit for 1 minute.
-Centrifuge for 1 minute and keep the eluate. It is ready for immediate use or can be frozen at -20°C.
BR JM
Ran PCR - June 22, 2012 (17-1)
Goal:
Restart of PCR of Tar portion of the Chimeric Tar and EnvZ protein with alanine insert.
Results:
Ran the PCR using primers 193 and 194 following the standard Fusion protocol and using k12 E.coli as template and saved the results: (17-1)
Primer 194 has an Alanine insert. JA
Ran PCR - June 22, 2012 (17-2)
Goal:
Restart of PCR of Tar portion of the chimeric Tar and EnvZ Protein with aspartate insert.
Results:
Ran the PCR using primers 193 and 195 following the standard Fusion prtocol and using k12 E.coli as template and saved the results: (17-2)
Primer 195 has an Aspartate insert. JA
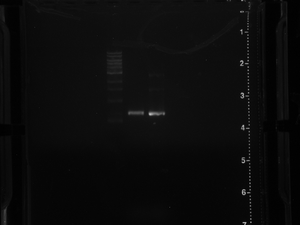
Gel of previous PCR - June 25, 2012 (17-3)
JM JA
Goal:
Get a band size: 600-700 base pairs
Lane order: Ladder, control, 17-1,17-2.
Results:
Perfect Bands for both.
JA
DNA Purification - June 26, 2012 (18-1)
Goal:
Purify PCR product with Alanine insert(17-1 PCR product tube).
Results:
Purified PCR Product using following protocol.Saved as(18-1).
-Insert mini spin column into collection tube and add 0.5 ml of column prep solution.
-Centrifuge for 30 sec to 1 minute.
-Add 5 volumes of binding solution to 1 volume of PCR mix and transfer solution to the binding column.
-Centrifuge for one minute.
-Discard the eluate and replace the binding column in the collection tube.
-Apply 0.5 ml of wash solution and centrifuge for 1 minute.
-Replace the column into the collection tube and centrifuge for an additional 2 minutes without adding any solution, discard the eluate.
-Place the column into a new collection tube and add 50 μl of Elution Solution. Let the tube sit for 1 minute.
-Centrifuge for 1 minute and keep the eluate. It is ready for immediate use or can be frozen at -20°C.
JA
DNA Purification - June 26, 2012 (18-2)
Goal:
Purify PCR product with Aspartate insert (17-2 PCR product tube).
Results:
Purified PCR Product using following protocol and saved as (18-2).
-Insert mini spin column into collection tube and add 0.5 ml of column prep solution.
-Centrifuge for 30 sec to 1 minute.
-Add 5 volumes of binding solution to 1 volume of PCR mix and transfer solution to the binding column.
-Centrifuge for one minute.
-Discard the eluate and replace the binding column in the collection tube.
-Apply 0.5 ml of wash solution and centrifuge for 1 minute.
-Replace the column into the collection tube and centrifuge for an additional 2 minutes without adding any solution, discard the eluate.
-Place the column into a new collection tube and add 50 μl of Elution Solution. Let the tube sit for 1 minute.
-Centrifuge for 1 minute and keep the eluate. It is ready for immediate use or can be frozen at -20°C.
JA
Ran PCR - June 26, 2012 (18-3)
Goal:
Second step of chimeric sewing PCR of Tar and Envz (Taz) with Alanine insert. This should create the Taz insert for the plasmid.
Results:
Ran the Fusion PCR using 18-1 as forward primer and 196 EnvZ reverse primer and saved the results: (18-3)
The PCR product used as a primer, 18-1, has an Alanine insert. JA JM
Ran PCR - June 26, 2012 (18-4)
Goal:
Second step of chimeric sewing PCR of Tar and Envz (Taz) with Aspartate insert. This should create the Taz insert for the plasmid.
Results:
Ran the Fusion PCR using 18-2 as forward primer and 196 EnvZ reverse primer and saved the results: (18-4)
The PCR product used as a primer, 18-2, has an Aspartate insert. JA JM