User:Stephanie P T. Yiu/Notebook/Identifying candidate colonies in molecular level/Entry Base
<html> <body>
Identifying Candidate Colonies in Molecular Level
</body> </html>
Authors
- Cheng, Karen K.O.
- Tam, Phoebe L.F.
- Tam, Sabrina K.M.
- Tang, Mandy L.Y.
- Siu, Mona M.Y.
- Yiu, Stephanie P.T.
Abstract
This training is to recap the basic steps and procedures of making constructs, such as digestion, ligation and transformation. In this training,constructs is made by replaceing mRFP with GFP in the parental plasmid. Both plasmid was digested with XbaI and SpeI. Hence, in the product, five different possible contructs may resulted: desired constructs, contructs with wrong insert orientation, contructs with two backbones, contructs with two inserts and self-ligated plasmids. The aim of making these constructs is to use methods indentifying the candidate colonies with the desired sequence. Colony PCR have been chosen to indentify the colonies. To distinguish the orienation, reverse primer and VF2 have been used. While distinguish between self-ligated plasmid and the constructs with two backbone or inserts, VF2 and VR primers are used. After running the PCR products in gel eletrophoresis, the one showing the expected band size are concluded having the desired contruct.
Introduction
Colony PCR can be used to check whether the constructs contain the desired insert or not. There are three main stages for the colony PCR, including denaturation step, annealing step and extension step. Plasmid DNA can be released to act as the template for amplifying the targeted DNA sequences during the denaturating step (initial heating step) while the oligonucleotide primer hybridize to the single DNA strands during the annealing step. For the extension step, engineered DNA polymerase will add complementary bases to the single DNA strands to build double-stranded DNA. These efficient steps can actually lift the throughput of colony PCR. Aside from this, colony PCR can also be used to determine the insert orientation and check if there is self-ligation by inserting specific primers which can provide the information on the molecular size. Meanwhile, no PCR amplicon exists unless the orientation is correct.
These properties can be made use of in this investigation to identify candidate colonies in molecular level. Two different pairs of primers were applied to produce two sets of results separately for checking the orientation and self ligation after digestion and ligation.
Methods and Materials
To study the methods of identifying candidate colonies in molecular level, a piece of DNA insert containing a GFP generator and another plasmid backbone containg a mRFP gene were restricted at XbaI and SpeI. The oligoes were then ligated and transformed. To verify the success of ligation, a colony pcr was performed followed by gel electrophoresis. Then, the results are compared with negative and positive controls to select cells containing the target DNA sequence.
2 sets of similar experiments were carried out.
The concentration of BBa_J23102 (with plasmid pSB1C3) and GFP generator were accessed by NanoDrop 2000 spectrophotometer. Approximately 2000ng of BBa_J2310 was digested with 0.2ul of XbaI (NEB, R0145L) and 0.2ul of SpeI-HF (NEB, R0140L) in CutSmart buffer. Similarly, approximately 6000ng of GFP generator was digested with 0.2ul of XbaI (NEB, R0145L) and 0.2ul of SpeI-HF (NEB, R0140L) in CutSmart buffer.Reactions were incubated at 37˚C for 1.5 hour. DNA samples obtained from digestion were analyzed by running 1% agarose gel electrophoresis with 120V electricity for 45 minutes, which was pre-stained by Midori Green for visualization of DNA fragments. Insets were extracted out from the gel and purified using Favorgen FavorPrep™ GEL/PCR Purification Mini Kit.
The digested BBa_J23102 and GFP generator were then ligated with 0.5ul T4 DNA ligase, in 10X T4 ligase buffer. Reactions were incubate at room temperature for 1 hour and underwent transformation, followed by overnight incubation.
Rationale
XbaI and SpeI restriction enzymes were being used for digestion, therefore, sticky ends of XbaI and SpeI were made. As XbaI and SpeI has a similar restrction sequence, it is likely for them to have ligations, which may in turn results in self-ligation and reverse orientation . In order to identify the candidate colonies, colony PCR was performed, and gel electrophoreiss was then adopted for identifying the candidate colonies.
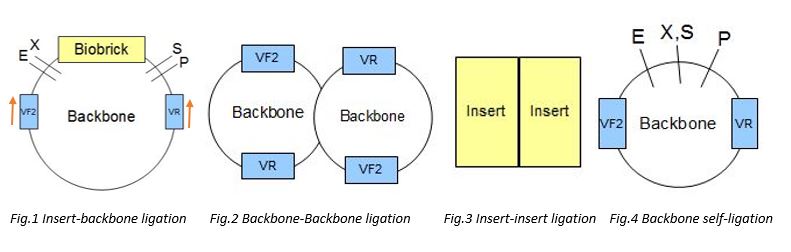
For checking self ligation Primers of VR and VF2 were being used. The two primers would bind to the corresponding VR and VF2 binding sites on the backone, and amplify all the seqeunces in between the VR and VF2 sites. (Fig.1) There would be 4 possible outcomes, including backbone-backbone ligation (Fig.2), insert-insert liagtion (Fig.3), backbone self-ligation (Fig.4) and the insert-backbone ligation (Fig.1). First, for backbone-backbone ligation, as there are only 2 backbones ligate with each other without the insert, the VR and VF2 primers would only amplify the sequences of the biobrick prefix and suffx, as well as a few sequence in between the VR and VF2 sites, therefore, after gel elcectrophoresis, the expected band size would be around 300-400bp. Second, for insert-insert ligation, as the two inserts ligate with each other without the backbone, there would be no VR and VF2 sites and so, the primers would not bind to it and wouldnot have amplification. Therefore, after the gel electrophoresis, the expected band size would be 0bp. Third, fr the backbone self ligation, as the backbone ligate with itself without the insert, the VR and VF2 primers would only amplify the sequences of the biobrick prefix and suffx, as well as a few sequence in between the VR and VF2 sites, therefore, after gel elcectrophoresis, the expected band size would be around 100-200bp. Forth, for backbone-insert ligation, after the gel electrophoresis, the expected band size would be around 2000bp.
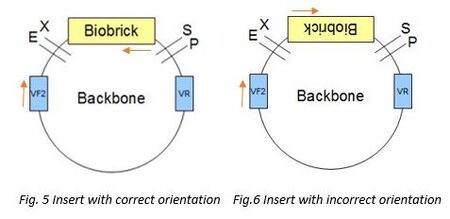
For checking orientation VF2 and the reverse primer of GFP were being used. VF2 primers would bind to the VF2 site on the backbone while the reverse primer will bind to the antisense of the GFP insert. The 2 primers would then amplify all the sequence between the insert and VF2 sites. There would be two possible outcomes, one with insert that is of correct orientation (Fig.5), another with insert of reverse orientation (Fig.6). First, for the product with insert of correct orientation, after gel electrophoresis, the expected band size would be around 1300bp. Second, for the product with insert of reverse orientation, as the two primers would not meet each other, as a result, there would be no amplification, therefore, after the gel electrophoresis, the expected band size would 0bp.