User:Sarah Burkhard/Notebook/471 Nano Notebook/2016/09/07
FluorescenceObjectiveDraw conclusions on reaction speed of nanoparticle formation from emission spectra taken in small time intervals of 5 minutes over a period of 3 hours. protein: BSA | pH: 4 Chemical backgroundBSA contains Tryptophan, which is fluorescent in the unfolded state. The excitation wavelength is 295 nm (more information read here http://www.physics.nus.edu.sg/~Biophysics/pc3267/Fluorescence%20Spectroscopy2007.pdf) With decreasing pH, the time it takes for BSA to unfold decreases as well. However, we still see an emission peak after the unfolding process: the newly formed gold nanoparticles are fluorescent. The time we see the emission peak around 435 nm. ProtocolPrepare solutions. Gold, protein, acid , water .
M1V1 = M2V2 was used for calculations (Anneliese's spreadsheet) A table of used concentration and volumes can be found here. Protocol for Fluorescence:
Preparation of stock solutions for UV-Vis:
Data AnalysisThe emission peak will shift to higher wavelengths, due to protein unfolding. The tryptophan is fluorescent with an emission peak between 300 and 350 nm. Gold particles are fluorescent as well, but differently as long as they are folded in the Protein (related to the low dielectric constant within the protein.)
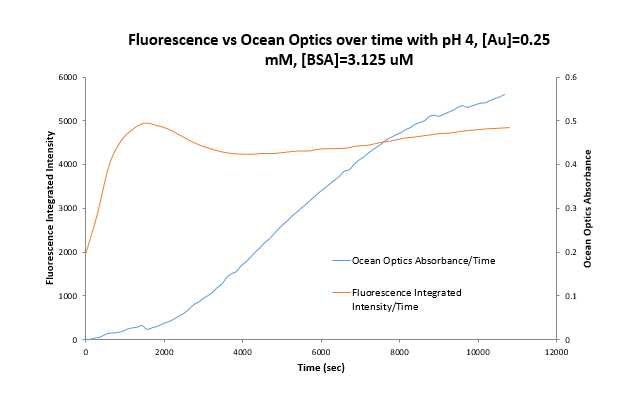
One can see that BSA unfolds and forms gold nano particles rapidly at pH 4 because emission at the relevant wavelength (~435 nm) increases drastically within the first 15 minutes. The emission maximum graph also shows us at what time the emission shifts which indicates the time the majority of protein is unfolded. The jump occurs between 5 and 10 minutes. A more detailed graph showing what happens in the first 40 minutes is here, a graph of intensity of emission vs time shows the emission peak and the following decrease:
NotesBSA stock solutions go 4 hours in oven, up to 80 C. menu > hill icon > menu > choose program 1 > 1 loop | |