User:Rebecca Brin/Notebook/Biology 210 at AU
2/6/14, lab 1 notes
Good work! Make sure you include pics from lab 1 and lab 2 by Sunday. Also, start working on building a map of your transect to detail your land and where your samples are taken from. We will talk about this more Wednesday.
AP
March 20, 2014
Embryology Lab
Objective:
Zebrafish are small living organisms that are often used for reasearch. This experiment was designed to see how abnormally high amounts of light exposure affect the development of the zebrafish. This includes their embryonic stages as well as juvenile phase.
Steps:
1.Place twenty healthy zebrafish larvae in a petri dish labeled “control”, and another twenty in a petri dish labeled “light”.
2.Place the control petri dish on a table, and place the light petri dish under a lamp that will be on 24/7.
3.On each day count:
a.Number of dead eggs
b.Number of living embryos
c.Number of living hatched fish
d. Number of dead hatched fish
4. Every day after day one make sure to removed empty eggs case/dead zebra fish and replace water.
5.When taking measurements (Days 2,5,7,9,12,14) use a representative sample to observe the following under a compound microscope. Put a few drops of water with the zebrafish in a depression slide.
a.body and tail pigmentation
b.eyes and eye movement
c.Heart and heart rate
d.pectoral fin development
e.size of yolk sac
f.development of swim bladder
g.development of mouth
h.general movement
6. On day 7, fix three samples from the control, and three sample from the light treatment group.
7.On day 14 make all the regular observations. In addition, use the fixed samples to measure tail length, entire length, and eye diameter.
Data:
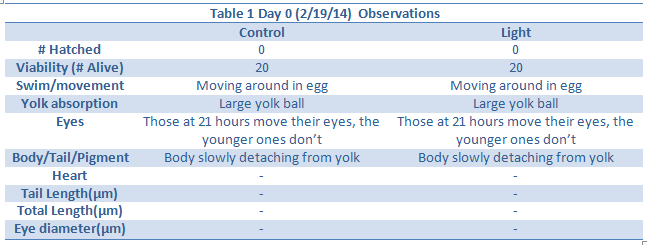
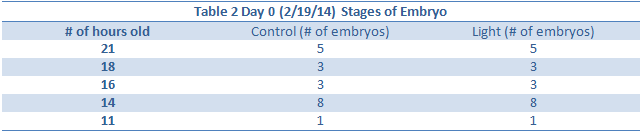
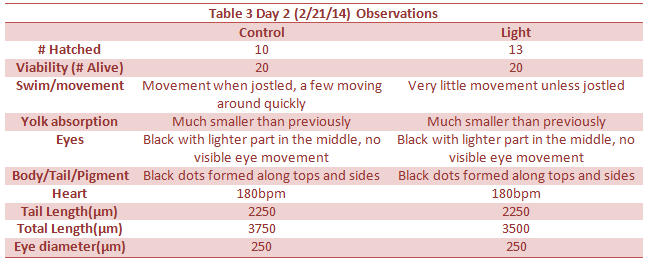
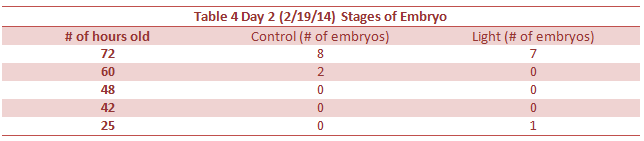
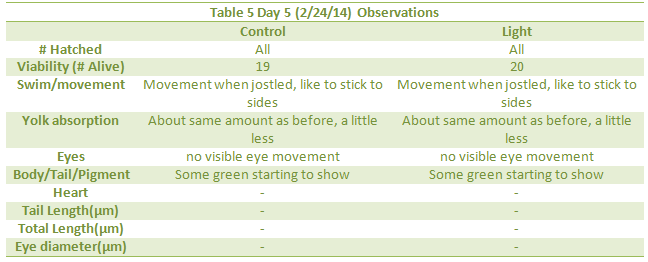
Heart, Tail Length, Total Length and eye diameter not taken because there were no depression slides.

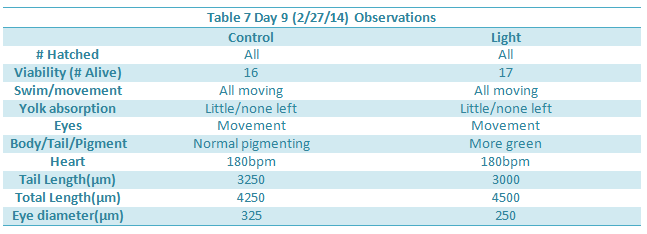
Day 12 observations could not be taken due to the snow day.
Day 14 – All but one zebrafish in the control were dead so data could not be collected. This may either be due to a sudden temperature change in the room, or a lack of food. However data was collected on this fish sacrificed last week.
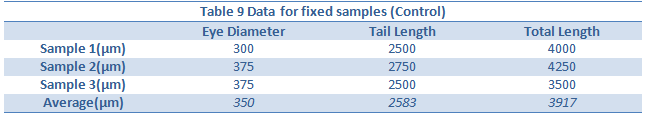
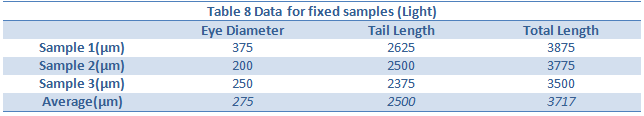
Conclusion:
The effect of an abnormally large amount of light was not enough to kill or severely impair the zebra fish. However there were a few notable differences in the between the control and experimental group. The differences began to appear one week into the experiment on day seven. On day seven it was seen that the treatment group moved around more than the control. This included their eyes moving around more often. In addition, they were thinner, possibly due to the constant movement. On day nine, it became clear the zebra fish in the light had more green pigments in their body as well. Lastly, by looking at tables 8 and 9, it can be seen that the tail lengths, body lengths, and eye diameter of the control group were greater than those of the light group. Because of the increased light, the zebrafish most likely did not stop moving around, as all the fish moved more when placed in light. Because of the increased movement, they used more energy and became smaller and slimmer.
RB
March 2, 2014
Data from Lab 6 (February 26, 2014)
Objective: DNA sequences from the PCR reactions were sent away to be sequenced. The purpose in this lab was to identify which bacteria were present in our transect.
Steps:
See Labs two and three
Results:

Note that the DNA sequence turned in during a previous lab from this transect was not returned so both of these samples are what other groups observed in the transect. There is no way to compare these to the bacterial samples sent in, as they were not sent in by my group.
Conclusion:
A wide variety of bacterium can be seen in our transect.
RB
February 26, 2014
Data from Lab 5 (February 12, 2014)
Objective:
Today, the Berlese funnel will be taken down, and invertebrate samples will be observed and characterized. After this, information will be gathered on what types of vertebrates could be occupying the niches.
Steps:
Part 1 – Observing acoelmates, psuedocoelmates, and coelmates
1.Observe the planaria slides, and live planaria, paying close attention to their movement.
2.Next observe the nematode slides, and live varieties, again paying attention to movement.
3.Lastly look at the live earthworms (coelomate) and observe their movement as well.
Part 2 – Analyzing Invertebrates from Berlese funnel
1. Take down the berlese funnel, and transfer it’s contents into two petri dishes, one for the top of the solution, the other for the bottom.
2. Try to identify five invertebrates from you funnel. Draw and describe each.
3.Observe the five jars with invertebrates and characterize them based on their morphology.
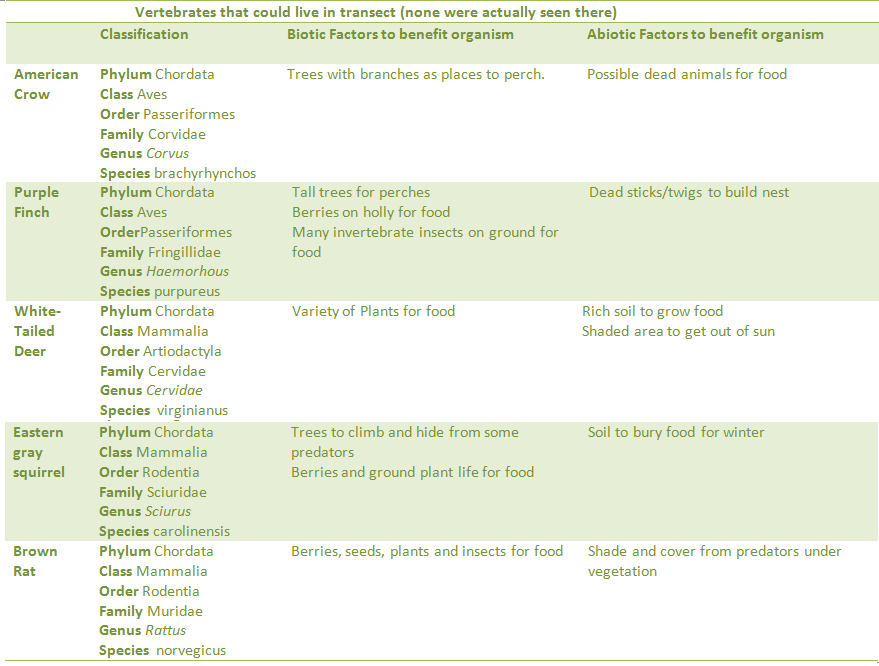
Part 3 – Vertebrate and Niches
1. Using the textbook, find five animals that might live in your transect. At least two should be birds.
2. Classify each, and then determine biotic and abiotic factors that lead you to believe they would inhabit the transect.
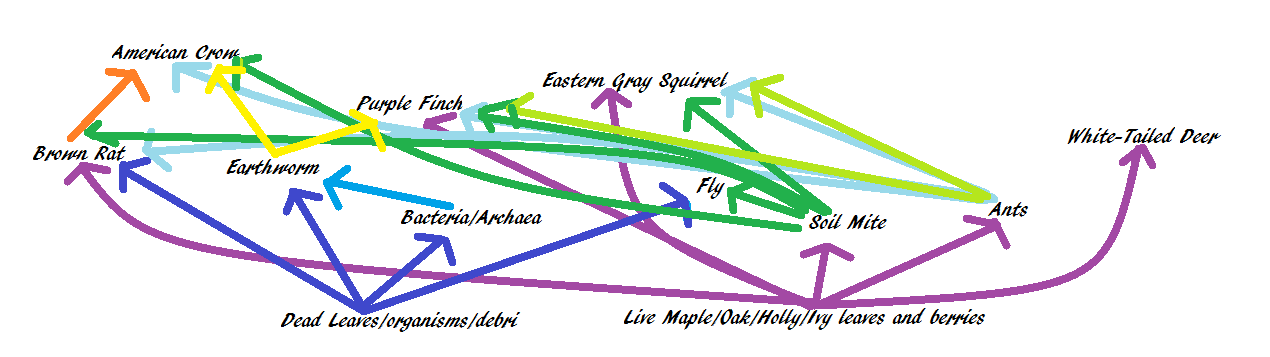
3. Lastly, construct a food web based on the organisms you have observed in your transect.
Data:
The Planaria have a wave like movement similar to slugs, and does this by moving its cilia. Nematodes differ as their movement is squiggly from side to side like a snake by contracting and relaxing their muscles. The earthworms have the most unique movement and move by stretching and contracting. They are able to do this do to the setae on their bodies.

The Largest of these invertebrates was the biting lice, but that was not found in our transect. The largest invertebrate in the transect was the fly, while the smallest was the soil mite. While the soil mite was only tenths of a mm in length, the fly appeared 2-3mm in length and was visible to the naked eye. There were a number of soil mites, while all the other invertebrates were only seen once. The mites seem to be the most prevalent.
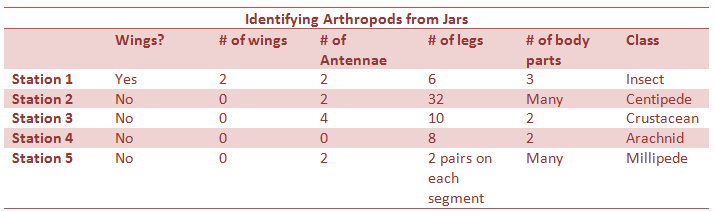
Conclusion:
Of the organisms obtained from the Berlese funnel four were identified. These organisms were a fly, an ant, a soil mite, and a flatworm. All of these came from a leaf litter sample taken from the front of our transect. Research then determined possible vertebrates living in the transect, and then a food web was constructed to determine how they interact. Every organism is an important part of the food web.
RB
February 24, 2014
Data from Lab 4 (February 5, 2014)
Objective:
Each transect is composed of various biotic and abiotic factors. Here the focus is placed on plants and fungi. Plants will be observed and collected from the transect, and fungi will be observed.
Steps:
Part 1 – Collecting samples from transect
1. Bring three bags to transect.
2. Obtain a leaf litter sample from transect. Dig about 500g of dirt mixed in with leaves and place in your bag. This will be used to set up Berlese Funnel.
3. Take samples of five plants (without harming them)
4. Also bring back seeds/pinecones/flowers if available.
5. Use this information to describe and identify them.
6. Take note of the vascularization of each plant.
7. Look at seeds/berries/venation of leaves to determine whether the plant is monocot or dicot and note other ways in which it may reproduce.
Part 2 Fungi
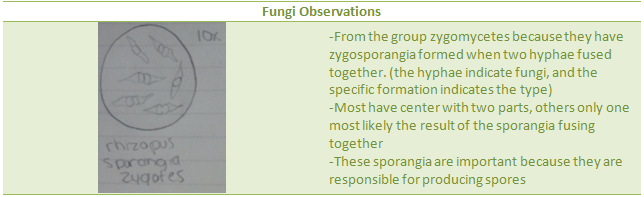
1.Look at fungal samples under dissecting microscope and decide which group it belongs to.
2.Draw a picture and describe it, explaining why you think it is a fungus.
Part 3 Berlese Funnel Set Up
1. Pour 50mL of 50:50 ethonol/water solution into a large test tube.
2. Put a screen at the bottom of a funnel and tape the sides of the screen to the funnel so it stays in place.
3. Place the neck of the funnel into the test tube.
4. Put the leaf/soil sample into the funnel.
5. Lastly put a 40 watt lamp above the funnel with the bulb 1-2 inches from the top.
6. Cover with foil.
Part 4: PCR
1. Load PCR gel with bacteria prepared from last lab.
2. Let PCR Gel run, and record results.
Data:
Locations of plant samples:


Also, PCR was performed on bacterial colonies from last lab and only one worked. Below is the gel electrophoresis. The bacterial colony from last lab from the -5 nutrient agar with tertracycline is in lane 4 at the 300-400 base pair mark.

Conclusion:
Samples were collected and analyzed to reveal black oak, sugar maple, ivy, holly tree, and eastern white pine. While the holly and ivy were still thriving, the oak and maple trees had lost most of their leaves. The pine had also lost some of it’s needles. Next Lab invertebrates from the Berlese Funnel will be examined.
RB
February 12, 2014
Data from Lab 3 (January 29, 2014)
Objective:
Bacteria are a diverse group of organisms with differing characteristics. Some are resistant to antibiotics, while others are killed by it. But all bacteria contain DNA, and the sequences can be used to identify the species. Using PCR, the DNA sequences of bacteria will hopefully be isolated.
Steps:
1. Check on Hay Infusion Culture and note any visible change.
2. Get agar plates and count number of colonies on each. Record in Table.
3. Pick three bacterial colonies from different agar plates.
4. For each:
a. Scrape up a tiny amount of the bacterial colony.
b. Put on slide.
c. Add a drop of water and the place a coverslip over the bacteria.
d. Observe at 10x and 40x and describe.
5. Using these same bacterial colonies, prepare gram stained slides by…
a. Label the slide.
b. Scrape a tiny amount of the bacterial colony and place on slide.
c. Pass bacteria through a flame three times bacterial side up.
d. Cover slide with crystal violet for 1 minute. Rinse the stain off with water.
e. Cover slide with Gram’s iodine mordant for 1 minute. Rinse gently.
f. Decolorize by covering with 95% alcohol for 10-20 seconds then rinse.
g. Cover slide with Safranin stain for 20-30 seconds. Rinse.
h. Blot excess water with paper towel and then let air dry.
6. Observe slides under 10x, 40x, and 100x oil immersion. Write down observations and sketch.
7. Pick two bacterial colonies from agar plates and prepare for PCR by…
a. Transfer small amount of colony into 100 microliters of water.
b. Incubate for ten minutes at 100oC
c. Use five microliters of supernatant in PCR reaction
Data:
Hay Infusion Culture – The cultures stench lost its potency, while some of the water evaporated, leaving more of the pine needles sticking out of the water. The change in stench is most likely due to the fact the cover was left off, and the odor escaped the container. This water level decreased sure to the waters evaporation, and left the remaining water with a higher concentration of bacteria/plants/fungi due to the decrease in volume.
Bacteria Cell morphology -
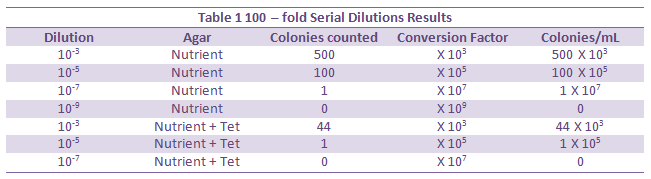
The plates without tetracycline have a much higher number of colonies. All of the species that were unaffected were able to grow on the tetracycline plates, while those without resistance were able to grow. The size of the colonies on the plate with tetracycline is noticeably larger than those on the regular agar plate.
No Archaea grew on the agar plates because Archaea for the most thrive only in extreme environments (extreme temperature, extreme salt concentration, etc.).
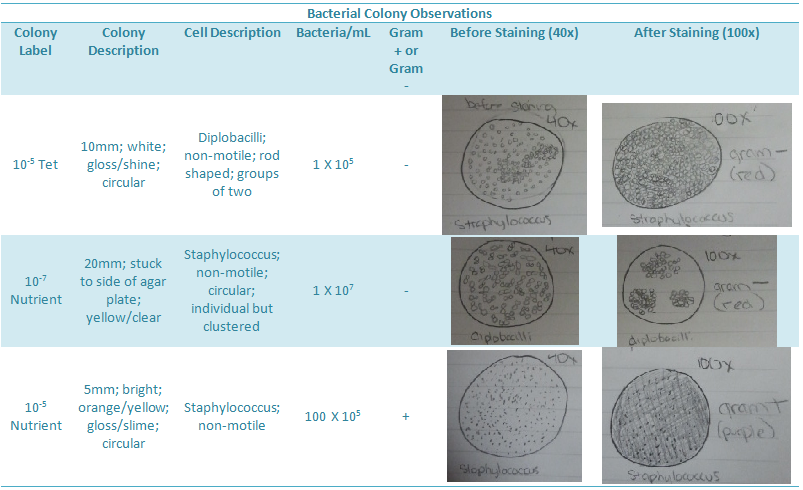
It should also be noted that the bacteria used for PCR reaction were from the colonies above. One was the colony on the -7 nutrient agar plate and the other was the colony on the -5 tetracycline plate.
Conclusion:
The plate with antibiotic had a significantly lower number of bacterial colonies than the normal agar plate. A large portion of the bacteria could not grow in the presence of the antibiotic because they are not resistant. The presence of the tetracycline lowered the total number of bacteria and fungi. It is effective against a broad range of bacteria, and significantly destroys non-resistant bacteria. This is because tetracycline binds to the 30S ribosome and inhibits it’s function. This stops proteins from being made and stops the bacteria from reproducing. In addition, tetracycline sometimes binds to the outside of a cell, change its shape and contorting the cell so there is a way for materials to slip out (Klajn, 2014). Next Lab the results of PCR will be analyzed.
Work Cited:
Klajn, R. (2014). Tetracycline - Antimicrobial properties. Retrieved February 11, 2014, from http://www.chm.bris.ac.uk/motm/tetracycline/antimicr.htm
RB
February 7, 2014
Data from Lab 2 (January 22, 2014)
Objective:
The are many types of protists. In order to determine the difference, a dichotomous key will be used to identify protists, and observations and characteristics of each will be observed using Hay Infusion samples.
Steps:
Part I (Practice with dichotomous key)
1. Obtain a known organism and observe characteristics including size, movement, and shape.
2. Obtain a dichotomous key and use it to characterize the organism. Confirm your decision by looking at the diagrams.
Part 2 (Hay Infusion Culture Observations)
1.Bring the Hay infusion culture to table without disturbing it.Make note of how it looks and smells.
2. Take samples from different areas of the culture (near and away from plant life). Note where each organism came from.
3.Put each sample on a slide covered by a cover slip.
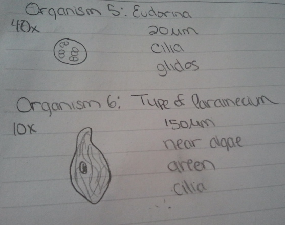
4.Draw pictures and observe 6 total organisms from varying locations. Also observe their size.
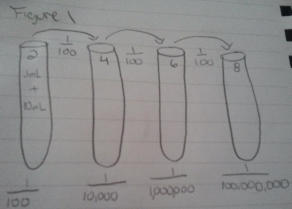
Part 3 (Preparing and Plating Serial Dilutions)
1.Get 4 test tube each with 10mL of sterile broth and label 2, 4, 6, and 8. Also get a micropipette set to 100 microliters.
2. Get four nutrient agar, and three tetracycline places. Label each of the places (nutrient 10^-3, 10^-5, 10^-7, and 10^-9/ tetratcycline 10^-3, 10^-5, and 10^-7)
3.Mix Hay Infusion Culture and take 100 microliters and add to test tube labeled 2. Mix this test tube and then add 100 microliters of this to tube 4.
4.Mix and add 100 microliters to tube 6.
5.Mix and add 100 microliters to tube 8.
6. Take 100 microliters from the test tube labeled 2 and put in onto the -3 agars. Take 100 microliters from the test tube labeled 4 and put in onto the -5 agars. Take 100 microliters from the test tube labeled 6 and put in onto the -7 agars. Take 100 microliters from the test tube labeled 8 and put in onto the -9 agar.
7. Spread the nutrient agar starting with the –9, and moving down to the -3. Spread the tetracycline agar starting at -7 and moving down to -3.
Observations/Data:
Hay Infusion Culture-

Brown/muddy with algae on top of water; Smells potent, like mud; leaf and pine needles sticking out of water; sediment on bottom of jar
Organisms one and two were taken from the bottom of the culture. Three and four were taken from near a leaf. Four and five were taken from near a different leaf in the culture. All of the organisms above are mobile protozoa. The Euglena, Eudorina, Pandorina, and Spirostomum were green containing chloroplasts, and appear to photosynthesize. All the organisms were alive because they meet the needs of life. For example, the Euglena is composed of a single cell which is organized to perform specific functions. It uses energy from the sun for photosynthesis and responds to its environment, staying away from other protists and adapting to its environment. Its size varies as it is able to grow, and can reproduce through fission.
Also most protists were found next to small pieces of algae on the slides, and seemed to stay near them for long periods of time. It could be that these organisms depend on the algae for food, or need nutrients from the algae to survive. Organisms that live away from plant matter don’t need this to survive, and therefor do better one their own. However, the majority of protists live near plant matter because they need these advantages, and can’t survive without them.
Conclusion:
By using a dichotomous key, three slides prepared using the Hay Infusion Culture could be used to identify six different organisms. These organisms were Euglena, Eudorina, Pandorina, Spirostomum, Paramecium bursaria, and another species of paramecium. The organisms were for the most part fairly large. This is due to the fact that in the Hay Infusions there was competition for nutrients and food, and some protists may have even consumed smaller protists. This competitive nature affected the contents of the culture.
In addition, the Hay Infusion Culture had only been sitting out for a week at this point. Had it been sitting out for longer, the odor would have been more potent in a closed container and there would be less life inside the culture, while the life that remained would be larger.
RB
January 30, 2014
Data from Lab 1 (January 15, 2014)
Objective:
A niche is selected for observation and future inspection. Both biotic and abiotic factors will be noted about the transect, and observations will be taken to define this niche. A Hay infusion will be prepared to aid in further investigation.
Steps:
1. Go to marked twenty by twenty foot section which from here on out will be the transect.
2. Take note of both the general characteristics as well as biotic and abiotic factors of the transect.
3. A 50mL sample of soil/leaves/ground material is placed into a tube to represent the transect.
4. 11.99g of this sample was added to 500mL of water, with .1gm of dried milk
5. The contents of the jar were mixed, and then the cap was removed.
6. The jar was labeled and left in the lab to be inspected later.
Data:
Transect Information
Location - At bottom of hill near church on Massachusetts Avenue
Topography - Neat bottom of hill, but slightly slanted; shaded
Biotic component- Large pine tree with trunk approximately two ft in diameter, ivy covering the ground, a few pine cones, maple tree, holly tree with a few red berries
Abiotic Components- soil, water (ground is damp), brown pine needles (fallen), maple leaves (fallen), pine cones (fallen)
Conclusions:
The transect was located in an area prone to both shade and water collection. The ground was covered in ivy and pine needles, making the soil beneath it a place small microorganisms would like. The Hay infusion culture created a place for small forms of life to grow and live over the coming weeks when they will be observed more closely. All in all, the transect was observed, and a Hay Infusion was done to be used in later labs as a source of microorganisms from the transect.
RB
January 22, 2014
Successfully entered first entry
RB