May 29, 2013
Reacting thiol DNA with ThT
Procedure
from Allison Alix 04/05/2013<br.>
- Combine 200μL of 5μM ThT (from AA 04/02/13) and 200μL 1.455μM thiol DNA
new ThT concentration in solution:<br.>
5μM(200×10-6L)=0.001μmoles<br.>
0.001μmoles/(400×10-6L)=2.5μM<br.>
<br.>
thiol DNA concentration to 1.455 from 53.1:<br.>
(53.1μM)V=(1.455μM)(200μL)<br.>
V=5.48μL 53.1μM thiol DNA in 194μL filtered water(?)<br.>
<br.>
new thiol DNA concentration in solution:<br.>
1.455μM(200×10-6L)=2.91×10-4μmoles<br.>
2.91×10-4μmoles/(400×10-6L)=0.7275μM=727.5nM<br.>
<br.>
- Heat at ~75°C for 25 minutes
- Allow solution to cool to room temperature
- Take absorbance and florescence measurements
Data
Absorbance
Peak observed at 414.00nm

Florescence
Excited at 414nm; peak observed ~500nm

Reacting AuNPs with thiol DNA/ThT Solution
Procedure
from Allison Alix 04/15/2013<br.>
re-do of 05/28/2013<br.>
- Combined 250μL 2.5μM ThT/727.5nM thiol DNA with 250μL 4% TEA with 100mM DTT, both made 05/29/2013
new DNA concentration:
727.5nM(250×10-6L)=0.181875nmoles<br.>
0.181875nmoles/(500×10-6L)=363.75nM<br.>
new ThT concentration:<br.>
2.5μM(250×10-6L)=0.000625<br.>
0.000625/(500×10-6L)=1.25μM<br.>
- Allowed to react for 10 minutes
- Extracted DTT with 4, 2mL aliquots of ethyl acetate, removing the ethyl acetate layer between each aliquot
- DTT dissolved in ethyl acetate layer (top)<br.>
- Combined thiol DNA/ThT and TEA with AuNPs and citrate buffer
- 1 AuNP:75 DNA ratio in solution<br.>
- Combined 232.4μL 1.25μM ThT/363.75nM thiol-DNA with 232.4μL 4.85nM AuNP and 35.2μL sodium citrate buffer
- Allowed to react for 10 minutes
- Redispersed in 500μL 50mM HEPES buffer before each of 4 rounds of centrifuging
- First round: centrifuged at 10000rpm for 14 minutes, dispered in 200μL HEPES buffer, then 12000rpm for 7 minutes
- Removed top layer (since no separation observed), redispersed in 300μL HEPES buffer
- Second round: centrifuged at 12000rpm for 12 minutes
- Removed top layer (since no separation observed), redispersed in 300μL HEPES buffer
- Third round: centrifuged at 12000rpm for 12 minutes
- Saved all supernatants
Data
Absorbance
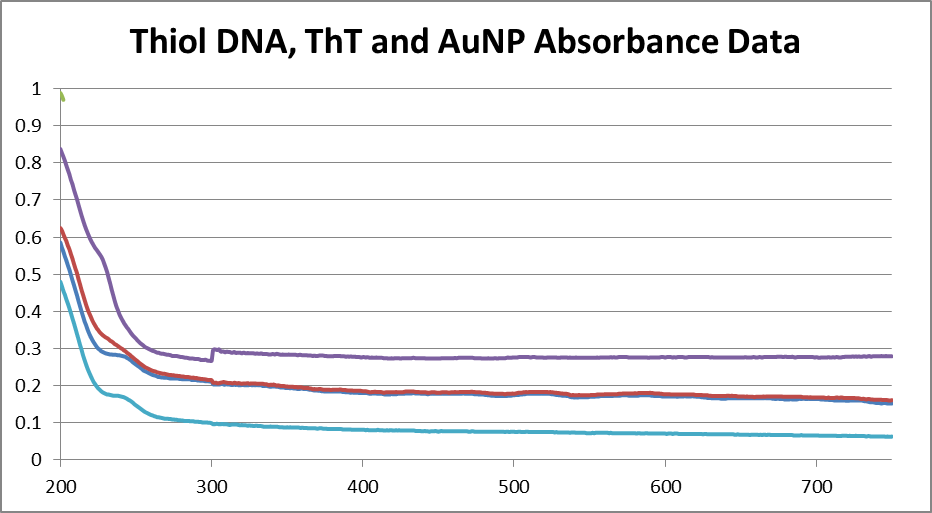 <br.>
Dark Blue: Water<br.>
Light Blue: Thiol DNA, ThT, AuNP solution<br.>
Red: Supernatant 1<br.>
Green: Supernatant 2<br.>
Purple: Supernatant 3<br.> <br.>
Dark Blue: Water<br.>
Light Blue: Thiol DNA, ThT, AuNP solution<br.>
Red: Supernatant 1<br.>
Green: Supernatant 2<br.>
Purple: Supernatant 3<br.>
Observations
Again, the extracted sample did not separate in the centrifuge as expected. I centrifuged as usual (though with larger time intervals and higher speeds) and removed the top "supernantant" layer despite the lack of separation. I took absorbance measurements, with inconclusive results.
| 

