User:Laura Flynn/Notebook/Experimental Biological Chemistry/2011/09/13
| Customize your entry pages | |
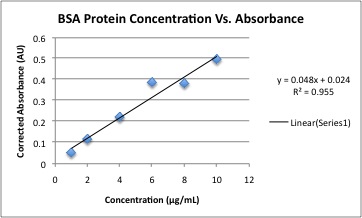
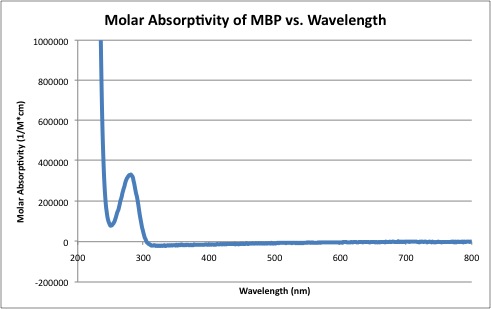
ObjectiveThe purpose of this experiment was to perform a Bradford Assay to determine the unknown concentration of maltose binding protein (MBP) using standards of bovine serum albumen (BSA). DescriptionA BSA solution with a concentration of .146μg/mL was used to create 1 mL standard solutions with water, 200μL of Bradford reagent, and a concentration of BSA as follows: 10, 8, 6, 4, 2, and 1μg/mL. 10μL of an unknown concentration of maltose binding protein was diluted with 10 mL of distilled water. 200μL of Bradford reagent was mixed with 800μL of the diluted maltose binding protein solution. Each solution was mixed by vortexing. UV-visible absorption spectra were recorded for each of the stock solutions, the unknown protein solution, and each blank as needed. The resulting spectras were used to calculate the unknown concentration. Data analysis: • Create a graph of protein concentration vs. absorbance for the BSA standards. • Generate a linear regression line from the standards and obtain the line’s equation. R2 value should ideally be above 0.97. • From the absorbances of the unknowns, solve the standard equation to find the protein concentration. • Determine the molar absorptivity of the unknown protein. DataThe linear regression was used to determine the concentration of the unknown MBP sample. At 595 nm, the absorbancy of MBP was determined to be 0.077 AU. By using the linear regression line, th concentration of MBP was determined to be 1.074 mg/mL. Since the solution used in the experiment was 1000 times more diluted than the original stock solution, the original stock solution was determined to be 1.074 g/mL. Molar absorptivity of MBP was plotted against wavelength.
| |