User:Kelsea L. McDyre/Notebook/Biology 210 at AU
March 23, 2014
Zebra Fish Lab:
We are testing the effects light has on zebrafish development.
The first day of lab, we placed 22 zebrafish into two different petri dishes -- one as a control kept under normal conditions and one placed under a light (NEED TO FIND OUT WATTS). At this point in the lab, the zebrafish were, on average, at the 12 hour stage.
Maxi then checked in on the the zebra fish -- need to collect data from her.
The next week, 18 zebrafish were left under the light and 19 were left as a control. We fixed two living zebrafish at this stage.
THe following week, no zebrafish were alive under the light, and two were alive from our control petri dish. We measured each fish under the microscope, and the following data was collected:
Control (2/27): 140 ocular spaces Control (3/6): 160 ocular spaces Light (2/27): 140 ocular spaces
March 9, 2014
DNA was collected from bacteria in our transect. Out of the two samples we sent in, only one result came back as Pseudomonas stutzeri. This bacteria has the following characteristics: rod-shaped, motile, gram-negative, found in soil.
February 28, 2014
This lab was an abridged version of the original. We looked observed the invertebrates found in our burlese funnel. Our group found only one single insect, as shown in the images section.
Images:
Image 1: Insect collected from Burlese funnel
February 21, 2014
Lab 4 - Plantae and Fungi
Objectives
1. To understand the diversity and characteristics of plants
2. To understand the importance and function of fungi
Procedure 1:
Collect plants from transect and describe characteristics, leaves, vascularization, and reproduction
Procedure 2:
Observe mold under dissecting microscope and answer the following questions:
1. What are Fungi sporangia and why are they important?
Fungi sporangia can be found growing on mycelium of fungus. They are black, spherical structures that are important to the growth of fungus. They hold spores, and when sporangia open, the spores are released
2. Draw picture of one and describe it. Explain why it is a fungus.
The picture of black bread mold can be found under images. The hyphae filaments can easily be seen with many sporangia growing on them.
Procedure 3 Setting up the Berlese Funnel to Collect Invertebrates:
1. Pour about 25 ml of 50:50 ethanol/water solution into flask
2. Fit a piece of screening material into bottom of funnel so leaf litter doesn't fall into flask
3. Place funnel into neck of flask
4. Place leaf litter into funnel and place apparatus under 40W lamp about 1-2 inches from the top of leaf litter; cover everything with foil
5. Leave under lights for 1 week
Images: Plants Collected
Image 1: Plant 1 collected from the base of cattails at the center of the transect
Image 2: Plant 2 (cattail) collected from the center area of transect
Image 3: Plant 3 collected from the periphery of transect from bushes
Image 4: Plant 4 collected from the back, grassy area of transect
Image 5: Plant 5 collected from the front area of transect
Black bread mold under dissecting microscope
Data
The following table contains data for Lab 4: https://docs.google.com/spreadsheet/ccc?key=0AthwYaNsaziNdEVrRXU5T0dJaXh1V19tRWlWSFR2ZUE&usp=sharing
KM
February 15, 2014
Lab 3 - Microbiology and Identify Bacteria with DNA
Objectives
1. To understand the characteristics of bacteria
2. To observe antibiotic resistance
3. To understand how DNA sequences are used to identify species
Procedure 1: Quantifying and Observing Microorganisms
1. Note changes in smell or appearance of hay cultures
2. Count number of colonies of on each agar petri dish made from the serial dilutions of the Hay Infusion Culture, and fill out Table 1 (found in Data section)
Procedure 2: Antibiotic Resistance
Answer the following questions based Table 1 Data
1,2. Do you see any differences in the colony types between the plates with vs. without antibiotics? What does this indicate?
Yes, there is a difference. There are colonies on three out of four nutrient plates, and only 1 plate with antibiotic had colonies. This indicates that many of the bacteria in the mini marsh environment are not resistant to antibiotics. As the dilution of the hay diffusion decreased, no more colonies were to be found on the tet plates. The 10^-7 tet plate had 11 colonies.
3. What is the effect of tetracycline on the total number of bacteria? Fungi?
Tetracycline decreases the number of bacterial colonies present on the agar. Bacterial colonies were not found on 2 of the three plates. Fungus was found on the 10^-7 tet plate.
4. How many species of bacteria are unaffected by tetracycline
2 bacterial species were unaffected by tetracycline. 2 different bacterial colonies (different colors and sizes) were found on the 10^-7 tet plate.
5. Find out how tetracycline works and the types of bacteria that are sensitive to this antibiotic.
Tetracycline work on both gram-positive and gram-negative bacteria. They prevent protein synthesis in bacteria by interfering with the transcription process. Tetracyclines are bacteriostatic, meaning it stops bacteria from reproducing. Bacteria sensitive to tetracycline include Escherichia coli and Haemophilus influenzae. [[1]][[2]]
Procedure 3: Bacteria Cell Morphology Observations
1. Observe prepared slides of bacteria to practice observing the cells
2. Prepare wet mount of bacteria from agar plates. Sterilize loop over flame and use it to scrape growth of agar and mix with a drop of water on slide. Place on cover slip and observe under microscope.
3. Prepare second slide, but smear a small amount of the colony in the drop over the surface to it can dry. circle area underneath the slide with wax pencil and use for the gram stain
4. Observe wet mount under 10x, 40x and determine shape and motility
5. Prepare a gram stain
a. label slides
b. heat fix the air dried slide by passing it through a flame three time with the bacterial smear side up
c. working with a staining tray, cover the smear with crystal violet for 1 minute, then rinse with water
d. Repeat step "c" with Gram's iodine
e. Decolorize by flooding smear with 95% alcohol for 10-20 seconds, rinse
f. Cover the smear with safranin for 20-30 seconds, rinse, and blot excess water
2. Observe under microscope under 10x, 40x, and the oil objective
Procedure 4: Start Preparation for DNA Sequence Identification
1. Select one colony from each of their agar plates (tet+ and tet-).
2. Transfer a single colony of bacter to 100μL of water in a sterile tube. Incubate at 100°C for 10 min and centrifuge
Miscellaneous Lab Questions
1. Do you think any archaea species will have grown on the agar plates?
No. Archaea species tend to be extremophiles. Our mini marsh environment does not offer such extreme conditions archaea species require (e.g. high temperatures, salty, etc).
2. Explain why the smell or appearance of hay infusion changes from week to week.
Microorganisms have time to reproduce, die, and digest organic matter. These various actions release gases with odors.
Data:
The following link contains Data from Lab 3: https://docs.google.com/spreadsheet/ccc?key=0AthwYaNsaziNdERfMW9taWJwZWNzd3ZrSjFwNFM1RHc&usp=sharing
Images
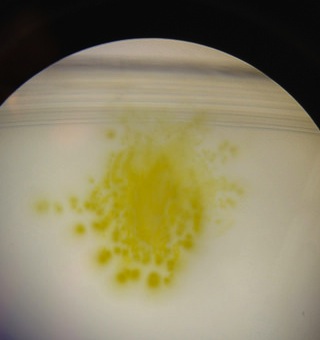
Image 1: Bacterial growth on 10^-5 agar plate. Under dissecting microscope.
Image 2: Bacterial growth on 10^7 agar plate. Under dissecting microscope.
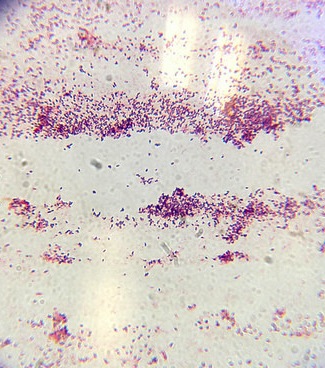
Image 3: Gram+ stain of bacteria from 10^7 agar plate. 40x objective.
Image 4: Gram +/- stain of bacteria from 10^-5 agar plate. 40x objective
KM
February 7, 2014
Lab 2- Identifying Algae and Protists
Objectives
1. To understand how to use a dichotomous key
2. To understand the characteristics of Algae and Protists
Procedure 1: How to Use a Dichotomous Key
1. Make a wet mount of the sample with the known organisms and observe with the microscope at 4X and then 10X
2. Focus on one organism and characterize it and record size
3. Obtain a Key that describes eight known organisms and use it to determine the identity the organism. Confirm with the diagram.
4. Repeat with a second organism.
Procedure 2: Hay Infusion Culture Observation
1. Observe hay infusion in its container and note smell, layers, apparent life, etc.
2. Make a wet mount of 2 different samples, each from a different niche in the infusion.
3. Observe slide and record size and appearance.
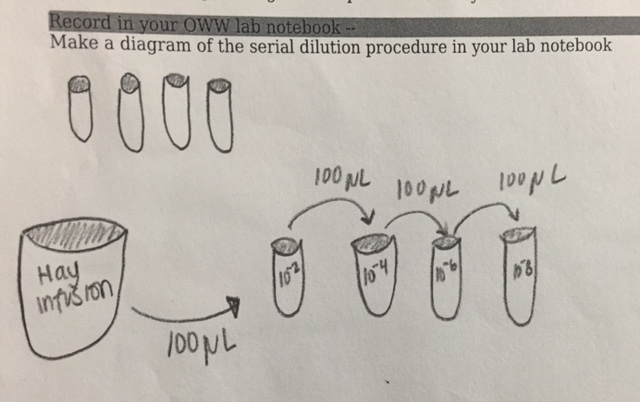
Procedure 3: Preparing and plating serial dilutions
1. Obtain four tubes of 10 mls sterile broth and label them 2,4,6,8.
2. Find four nutrient agar and four agar plus tet plates. Label one plate from each group 10-3, 10-5, 10-7, and 10-9
3. Swirl hay infusion to mix up all organisms and take 100 μL from the mix and aseptically add this to the 10 mls of broth in the tube labeled 2 for 10-2.
4. Take 100μL of broth from tube tube and inoculate tube 4. Swirl to mix
5. Repeat twice more for the final two dilutions.
6. Take 100 μL from the 10-2 tube and aseptically place on the surface of the nutrient agar plate 10-3; spread sample and repeate on tet and remaining paltes
7. Allow to incubate for one week
Data:
please refer to post made on January 23, 2014 for data regarding hay infusion organisms
Lab questions:
Any apparent life on surface of hay infusion? No
Why might organisms be near plants in infusion? Organisms dependent on oxygen would be found near photosynthesizing plants
If the sample was observed in another 2 months, what would you expect to find? I would expect to find growth on the top of our infusion. There was a film on the top after one week. I would expect to see more organisms, as well, specifically the organisms which are most fit to live in the mini marsh/hay infusion environment.
What selective pressures affected compositions of your samples? A lack of food would in the jar would act as a selective pressure in the hay infusion. Also, organisms that require oxygen to survive would not be thriving.
Describe an organism seen in hay infusion: Chlamydomonas are found in soil, stagnant water, and other areas including snow. They can reproduce sexually and asexually. They create their own food, for they are phototrophs. They have flagella, making them motile
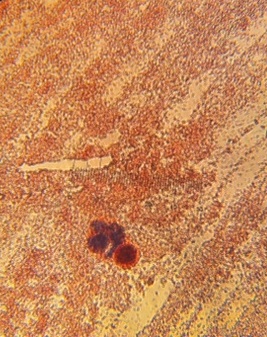
Image 1: Hay infusion of mini marsh sample
Image 2: Bird's eye view of mini marsh hay infusion, no apparent signs of life on surface
Organisms found on top layer of hay infusion, including chlamydomonas and an invertebrate
Figure 1: Serial dilution process
KM
January 31, 2014
Lab 1-Biological Life at AU
Objectives
1. To understand natural selection
2. To understand the biotic and abiotic characteristics of a niche
Procedure I: The Volvicine Line
1. Prepare a slide of living Chlamydomonas
2. Add protoslo to slide to slide to slow down chlamydomonas
3. Observe under microscope and record number of cells, colony size, functional specialization of cells, and reproduction specialization
a. look for stigma, chloroplast and the pyrenoid, and the flagella
4. Observe Gonium on a new slide and record number of cells, colony size, functional specialization of cells, and reproduction specialization
6. Observe Volvox on a slide and record number of cells, colony size, functional specialization of cells, and reproduction specialization
Hypothesis
Volvox will show more signs of development than Gonium and Chlamydomonas
Data
The following link contains data from Procedure 1 in Table 1 https://docs.google.com/spreadsheet/ccc?key=0AthwYaNsaziNdENxVElWLXY1ZEM4aXpMN0F6LWtHN0E&usp=sharing
Conclusions
The data from Table 1 supports the hypothesis; Volvox does have characteristics, like motility, oogamy, and large colony size, that shows it is more developed than Gonium and Chamydomonas.
Procedure II: Defining a Niche at AU
1. Set the 20 by 20 feet dimensions of the transect (either 1, 2, 3, 4, or 5) with 4 popsicle sticks
2. Use the sterile 50 ml conical tube to take a soil and ground vegetation sample. Make sure it is representative of the ground and what is on the surface of the ground.
3. Weigh 10 to 12 grams of the soil/ground sample and place in the plastic jar with 500 mls of deerpark water.
4. Add 0.1 gm dried milk and gently mix it all up for about 10 seconds.
5. Remove the top of the jar and place the open jar in a safe place in the lab.
Data
The data collected from Procedure 2 is found in the following link: https://docs.google.com/spreadsheet/ccc?key=0AthwYaNsaziNdEttRHh5Y1FqOWowY3p4YUIxdTRwbmc&usp=sharing
No conclusions can be drawn yet --Kelsea L. McDyre 09:58, 31 January 2014 (EST)
January 23, 2014
Hay Infusion Observations: faint "outdoors" scent, bottom layer contains dirt, leaves, branches, middle layer contains cloudy water, slight film on top of water and top of plant is floating
Sample 1: bottom layer - colpidium (very fast, tiny), chlmaydomonas
Sample 2: middle layer - colpidium (very fast, tiny), paramecium aurelia or paramecium bursaria, chlmaydomonas
Sample 3: chlamydomonas KM
January 22, 2014 Transect: Mini marsh
Location: Behind Kogod, facing Katzen
Topography: sloped, exposed to sunshine, shade from building, no trees, drain on edge of transect (at bottom of slope), sidewalk along another edge of transect, rocky, maintenance of AU affects area (e.g. fertilizers cutting grass)
Biotic factors: soil, cattails, grass, bushes, bugs, worms, squirrels, rats/mice
Abiotic factors: debris, large rocks, small rocks, drain, cement pieces KM