User:Haoxic
Bonding Surfaces
Introduction
Bonding surfaces is a process that bonds two surfaces to assemble them into a complete unit. Bonding Surfaces is a critical fabrication step for microfluidic divice assembly. While creating microfluidic devices, the devices usually contains multiple layers as shown in figure 1, and since the devices contain fluids inside, incomplete or low quality bondings may cause fluid leakage and directly affact the functionality of the devices. According to properties of the surfaces and the requirements for the devices, there are multiple methods to bond surfaces, and a some of the most common methods for bonding surfaces are UV/Ozone treatment, Thermal Bonding, Adhesives and Crosslinking.
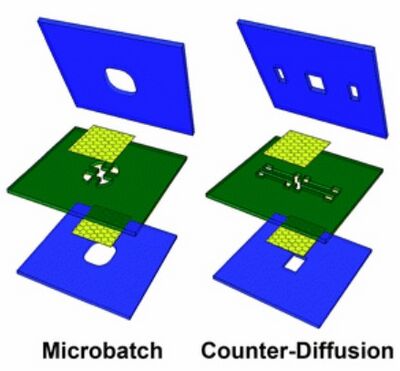
Methods to bond surfaces
Ultraviolet (UV)–Ozone Treatment
UV-Ozone treatment is also known as UV-Ozone Cleaning/Activating, which is a cleaning/activating process of sample surfaces used in material and device research. It is one of the popular techniques as well as oxygen plasma treatment. In this process, photochemical reaction happens on the surfaces of samples.
Ultraviolet light (UV) lamp irradiates two types of wavelength (185 and 254 nm). Each wavelength has different roles for chemical reaction. 185-nm UV light dissociates molecular oxygen O2 into triplet atomic oxygen O(3P). Triplet atomic oxygen O(3P) combines with molecular oxygen O2 and generates ozone O3.
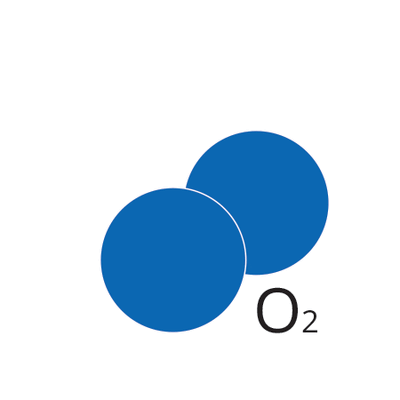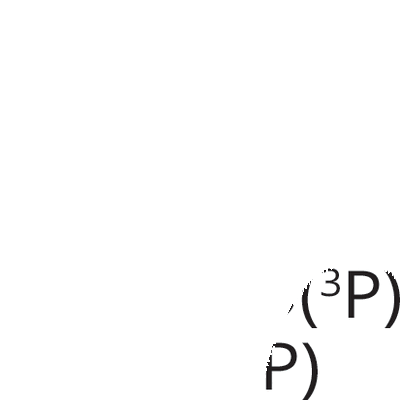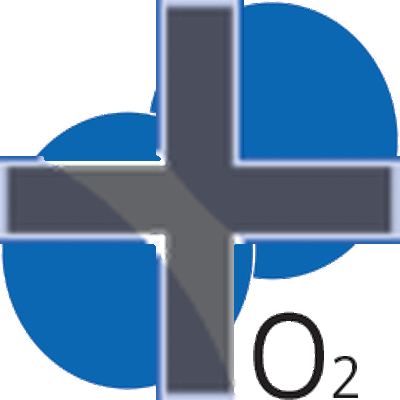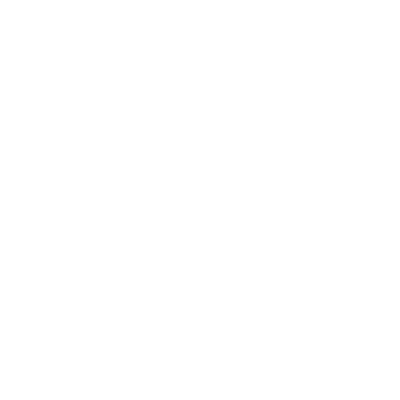
On the other hand, 254-nm UV light dissociates ozone O3 and forms molecular oxygen O2 and singlet atomic oxygen O(1D). Singlet atomic oxygen O(1D) has strong oxidation power, and it reacts with substrate surfaces. In this reaction, the surfaces were oxidized on inorganic substrates such as silicon wafers. In the case of organic materials, chain scission of molecules happens and organic residue contaminants are gently removed from the substrates as volatile byproduct molecules such as CO2, H2O and O2.
Poly(dimethyl siloxane) (PDMS) and poly(vinylmethyl siloxane) (PVMS) are two types of commonly used polysiloxanes. Both PDMS and PVMS SENs undergo dramatic changes in their properties when exposed to UVO. The surface chemical composition of both PDMS and PVMS at long UVO treatment times changes substantially and features a high density of hydrophilic groups.[3] These oxidized polysiloxanes will then be able to attach to inorganic surfaces such as silican wafers of glass slids to form conformal Si-O-Si bondings.
Thermal Bonding
Thermal bonding is a bonding between materials that their physical properties changes with increasing temperatures. These materials are usually called thermal plastics. Thermal plastics are materials that are reversibly moldable above a certain temperature, and solidify upon cooling. Thermal bonding occurs when thermal plastics are heated to and beyond their Glass transition temperature, and pressures are applied.
The temperature where the materials start becoming moldable is known as the Glass Transition Temperature. Glass transition is the reversible transition in amorphous materials from a hard and relative brittle state into a soften or viscous state when increasing the temperature.
Thermal bonding requires extremely clean and flat surfaces and is typically performed in cleanrooms. In application, thermal bonding can be used to bond glass to glass or silicon to silicon. In thermal bonding, similar materilas are bonded at elevated temperatures and pressure.[4]

Adhesives
An adhesive layer between surfaces can bond the surfaces onto each other. The adhesive is typically and epoxy, which can be either cured by exposure to UV light or by heating. The curing process results in bonding between the surfaces.[4]
Cross-linking
When the surfaces are photocrosslinking photoresists, they can bond to each other by cross-linking under present of light. Photocrosslinking photoresist is a type of photoresist, which could crosslink chain by chain when exposed to light, to generate an insoluble network. Photocrosslinking photoresist are usually used for negative photoresist. There are cross-linkers in the photocrosslinking photoresists that will be activated when exposed to light, the crosslinker ratios determine the rate of the bonding process. When there are more light sensitive cross-linkers in the photoresists, the the surfaces will bond to each other faster, and in opposite, if there are less light sensitive cross-linkers in the photoresists the bonding process will be slower.
Reference
[1]-S. Sui, Y. Wang, K.W. Kolewe, V. Srajer, R. Henning, J.D. Schiffman, C. Dimitrakopoulos, S.L. Perry, "Graphene-Based Microfluidics for Serial Crystallography," Lab on a Chip, (2016) 16, 3082-3096.DOI: 10.1039/C6LC00451B
[2]-SAMCO Inc. The Basics of UV-Ozone Cleaning of Surfaces
[3]-A. E. Özçam, K. Efimenko, J. Genzer, "Effect of ultraviolet/ozone treatment on the surface and bulk properties of poly(dimethyl siloxane) and poly(vinylmethyl siloxane) networks" Polymer, (2014) 55, 3107-3119.
[4]-Perry, S. ChE590E Microfluidics and Analysis. Lecture 2A: Microfabrication Processes. 2018. University of Massachusetts, Department of Chemical Engineering.
