User:Echia
Digital Microfluidics: Sciences behind it (BETA version - Final version please refer to WikiTextbook)

Introduction
Digital microfluidics technology allows users to control a very small volume of liquid and carry out the micro-reaction process, usually within microliters. On 2003, Sung Kwon Cho, Hyejin Moon, and Chang-Jin Kim have developed a complete idea of digital microfluidics at UCLA. The science behind digital microfluidics is by manipulating the surface tension of liquid by applying voltage. Thus, we need to understand:
- what is the surface tension of liquid
- what is electrowetting
One of the best demonstrations is MIT Media Lab's project named Programmable Droplets. Users could manipulate the droplets by moving them around, merging, and splitting them.

How water strider could walk on the surface of the water? Water-air interface exists a very special force, and this is known as surface tension. Surface tension exists is due to air-liquid interface: the liquid molecules on the interface are "preferred" to be pulled inward to a higher density liquid phase. Surface tension is considered as line force, which means there is only tensile force that exists on the interface.

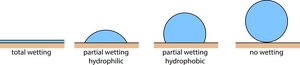
Above, we take the air-liquid interface to elaborate on the surface tension phenomenon. Actually, solid-liquid interface could also affect surface tension. In general, any phase reacts with a liquid interface that will engender surface tension. This will lead to either the liquid more likely to be attracted by solid surface (hydrophilic) or attracted towards inward of itself (hydrophobic).
Hydrophilic and Hydrophobic Surfaces
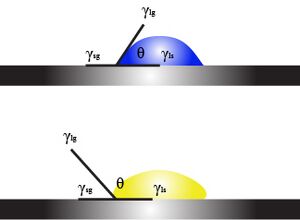
As mentioned above, the surface tension of liquid depends on the affinity between liquid molecules and a solid surface. The liquid that tends to "wet" the solid surface is said as hydrophilic, while in contrast is hydrophobic. For instance, from the image of the lotus leaf, the leaf surface doesn't get wetted by water and it is a hydrophobic surface. Different liquids they also have different terms to describe the surface phenomena: for oil, the surface that allows oil to wet it is oleophilic, else it is oleophobic. In 2014, Liu et. al. has successfully modified a surface that could repel any liquid, and this is the so-called "omniphobic" surface[4]. To quantify the affinity of liquid molecules to a solid surface, we measure the contact angle of the liquid and the surface tension can be quantified through Young's equation.From the free-body diagram on the right, we could come out with
[math]\displaystyle{ \gamma _{SL}=\gamma _{SG} -\gamma cos(\theta) }[/math]
This equation is applying force balance to hold a droplet on a solid surface. Young's equation is the governing equation describing surface tension force, and this gives a fair description of surface traction.
Summary of surface tension
To sum up, the surface tension of a liquid is phenomena that describe how liquid holds itself into a droplet. Young's equation has given an idea of how surface force could be mathematically formulated as a function of contact angle. In other words, if we could discover a method to manipulate the contact angle of liquid droplet, making a hydrophobic surface becomes hydrophilic, this is how we could manipulate a droplet movement. This method will be described in the next "Electrowetting" section.
Electrowetting
Electrocapilary and Lippmann's equation
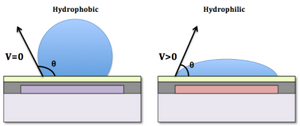
Electrowetting means that manipulating the liquid drop from the status of hydrophobic to hydrophilic. This phenomenon is discovered by Gabriel Lippmann in 1875[5]. At that time, he found the changes in mercury meniscus by applying voltage through the electrolyte. This is known as electrocapillary, and this occurs on liquid-liquid interface(electrolyte and mercury). Carrying this idea, if the surface tension can be manipulated on solid-liquid interface, this is the so-called electrowetting.
The Lippmann's equation is
[math]\displaystyle{ \gamma _{SL}(\phi)=\gamma _{SL}(0)- \frac{1}{2} c \phi ^2 }[/math]
Where we introduce a new parameter ϕ, which is the voltage potential agglomerate on the interface liquid and solid. The term γSL(ϕ) means that the surface tension is the function of potential at the solid-liquid interface, γSL(0) is surface tension under no voltage applied, c is the capacitance per unit area of electrical double layer, EDL (for electrowetting) or dielectric layer (for EWOD)[6].
Electrowetting has many applications such as microfluidic valves that have been presented in Electrowetting Valves - Davis Miller, Sarthak Saha (and they also have more comprehensive content about electrowetting that couldn't be covered in this topic like Wenzel wetting and Cassie wetting). In this section, we will introduce another application of electrowetting, digital microfluidics.
Combining Young's and Lippmann's finding --- Lippmann-Young Equation
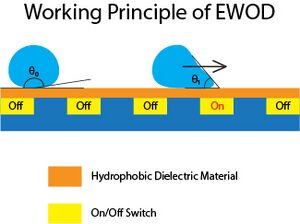
Lippmann-Young equation is the governing equation of electrowetting, which is also the model to describe EWOD that is the working principle of digital microfluidics. By substituting the γSL from Lippmann's equation to Young's equation, we yield:
[math]\displaystyle{ cos(\theta)=cos(\theta _{o})+ \frac{\epsilon V^{2}}{2\gamma _{LG}d} }[/math]
From the equation, we could design a huge array of dielectric board that could rapidly on/off to perform rapid EWOD, and by changing the electric voltage from array element to next element, the droplet will move to next array by "wetting" the element, while recede from the previous element at the same time due to the surface becomes hydrophobic again. This allows user to control a single droplet precisely with fixed tiny amount and allow microreaction to occur. [Baebies, Inc] has developed a microfluidic device that could precisely control each species to react with and in a programmable manner. This brings a huge convenience for micro-reaction.
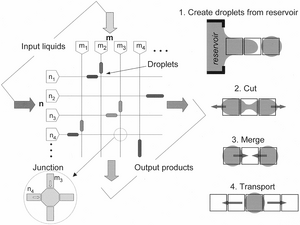
Basic Operations for Digital Microfluidics
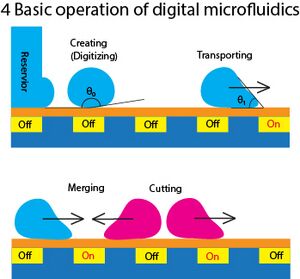
Now, we could take advantage of EWOD and start designing digital microfluidic devices. Digital microfluidic devices (DMF) can come in different patterns and designs to accommodate users' requirements. However, a DMF must consist of the following operations to qualify as a complete device:
Creating(Digitizing)
This is the most important step to discretize the input liquid into discrete droplet size (e.g. 10μL per droplet).
Transporting
Move the droplet around. The working principle of moving the droplet is simply switching on the target element in an array and the droplet will move towards it.
Cutting
Splitting the droplet into half. This can be achieved by switching on the nearby elements and the droplet will be pulled in two opposite directions.
Merging
When there are two different droplets that are attracted into same element, the droplets are now mixed together and create a larger droplet. This also starts micro-reaction if these two or more species could react together.
- Concept checkpoint: Recall the [Programmable Droplets] shown above, is it a "real" digital microfluidic device?
Applications
Constructing Heterogeneous Polymers by Varying Tiny Amount of Monomer and Crosslinker[7]
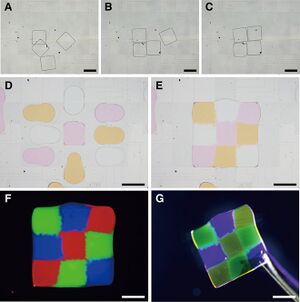
One of the best applications of digital microfluidics is to fabricate heterogeneous structured polymers with either different cross-linking agents for the same monomer or different polymer types into single pieces of product. This has been demonstrated by Chiang et. al. and then cells were cultured on the heterogeneous polymer surface to observe their growth.
Automated Molecular Biology Platform by Sandia National Lab[9]
Digital microfluidics is not only a benchtop in-lab technique, but it has also been commercialized and could perform many analyzing techniques that could only be carried in lab before, e.g. genome sequencing. This is known as "mobile lab" that only a small amount of sample is needed and it could perform analytic tasks while carrying it. This is beneficial for the rescue team, soldiers at the front line, remote areas to perform on-site testing.
References
- ↑ [1] Sung Kwon Cho, Hyejin Moon and Chang-Jin Kim, "Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits," in Journal of Microelectromechanical Systems, vol. 12, no. 1, pp. 70-80, Feb. 2003.
- ↑ [2]Gao, X., Jiang, L. Water-repellent legs of water striders. Nature 432, 36 (2004).
- ↑ [3]Hu, D., Chan, B. & Bush, J. The hydrodynamics of water strider locomotion. Nature 424, 663–666 (2003).
- ↑ [4]Science 28 Nov 2014:Vol. 346, Issue 6213, pp. 1096-1100 DOI: 10.1126/science.1254787
- ↑ [5] Mugele, Frieder, and Jean-Christophe Baret. "Electrowetting: From Basics to Applications." Journal of Physics: Condensed Matter 17.28 (2005): R705-74. CrossRef. Web.
- ↑ [6] Lee, Junghoon, et al. Electrowetting and Electrowetting-on-Dielectric for Microscale Liquid Handling., 2002. Web.
- ↑ [7] Chiang, Min-Yu and Hsu, Yao-Wen and Hsieh, Hsin-Yi and Chen, San-Yuan and Fan, Shih-Kang, "Constructing 3D heterogeneous hydrogels from electrically manipulated prepolymer droplets and crosslinked microgels" Science Advances 26 Oct 2016: Vol. 2, no. 10, e1600964
- ↑ [8] Chiang, Min-Yu and Hsu, Yao-Wen and Hsieh, Hsin-Yi and Chen, San-Yuan and Fan, Shih-Kang, "Constructing 3D heterogeneous hydrogels from electrically manipulated prepolymer droplets and crosslinked microgels" Science Advances 26 Oct 2016: Vol. 2, no. 10, e1600964
- ↑ https://ip.sandia.gov/technology.do/techID=102
