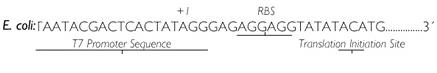User:Dirk Van Swaay/Sandbox
Front Page
Purpose
Audience
- Audience Characteristics
- Information Preferences
- Computer Specifications
- Web Experience
Experiment Planning
Objectives
- To roughly characterise the emulsion process
- Prepare emulsions from three suspensions:
- POPC/dodecane
- DOPC/dodecane
- Span80/dodecane
- Estimate volume of phase-separated aqueous solution at set intervals
- Intervals of 30 minutes
- Create five sample containers with fifths of the total aqueous emulsion volume under oil
- Collect 5μl emulsion samples at set intervals for observation in the microscope
- Intervals of 30 minutes
- Use light microscopy
- Know image volume
- Photograph random population samples (how many samples for significance?)
- Characterise mixing process
- Measure RPM of mixer
- Ensure all mixing is done at the same RPM
- Calculate surface area of glass container
- Estimate shear force (how????)
- Relate shear force and time to vesicle size and emulsion efficiency
- Prepare emulsions from three suspensions:
- To evaluate the efficiency of the interface transfer
- Measure vesicle density in emulsion
- Measure vesicle density in emulsion after centrifugation over the interface
- Measure vesicle density in solution
- Compare size of vesicles in all three measurements above
- To test Span80 emulsion on POPC and DOPC interface
- Form interfaces with POPC/dodecane and DOPC/dodecane
- Add Span80 emulsion to interface according to protocol
- To compare performance between suspensions with and without overnight incubation
- To assess the effect of circulation by 23 gauge syringe needle
- Take a sample without circulation, and look at it under the microscope.
- Take a sample with circulation, and compare to that without.
- Perform a rough test on gene expression within vesicles
- Carry out only two experiments, using POPC/dodecane 10ml emulsion, with and without interface
- Select construct to use and DNA concentration
- Prepare solution for encapsulation
- If induction is necessary, account for it in the process
Preparations
Desiccations
- 6x POPC 10ml
- 4x DOPC 10ml
Suspensions
- 3x POPC/dodecane 10ml
- 2x DOPC/dodecane 10ml
- 1x Span80/dodecane 10ml
Emulsions
- POPC/GFP
- DOPC/GFP
- Span80/GFP
- POPC/Cell Extract
- POPC/GFP 2ml
- DOPC/GFP 2ml
Interfaces
- POPC/dodecane for POPC/GFP
- POPC/dodecane for Span80/GFP
- POPC/dodecane for POPC/Cell Extract
- POPC/dodecane for DOPC/GFP
- DOPC/dodecane for DOPC/GFP
- DOPC/dodecane for Span80/GFP
- DOPC/dodecane for POPC/GFP
No interface
- POPC/GFP
- DOPC/GFP
- POPC/Cell Extract
- Span80/GFP
Samples to be taken
From solution
- POPC no interface
- POPC with interface
- DOPC no interface
- DOPC with interface
- POPC no interface CELL EXTRACT
- POPC with interface CELL EXTRACT
- Span80 no interface
- POPC:Span80
- DOPC:Span80
- POPC:DOPC
- DOPC:POPC
From emulsion (every 30mins)
- POPC/dodecane
- DOPC/dodecane
- Span80/dodecane
- POPC/Cell Extract
- POPC/GFP 2ml
- DOPC/GFP 2ml
Tomorrow
- Plan more of the experiments
- Plan schedule and prepare for the next day
The day after
- Run the cell extract experiment (one day protocol) as a pilot - not worrying about whether expression occurs before or after encapsulation
- Maybe run some of the characterisation experiments
Notes
- Decide which method to continue improving
- experiment on osmolarity
- Improve emulsion step
- Test different surfaces, volumes, speeds, and durations
- Collect samples at regular intervals to assess emulsion process
- calculate the volume of sampling area
- set up lab notebook template
- experiment aim
- protocol modifications
- samples taken
- data sought
- results
- comments
- propose shear calculations
Vesicle References
Reviews
- Noireaux V, Bar-Ziv R, Godefroy J, Salman H, and Libchaber A. Toward an artificial cell based on gene expression in vesicles. Phys Biol. 2005 Sep 15;2(3):P1-8. DOI:10.1088/1478-3975/2/3/P01 |
- Noireaux V and Libchaber A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc Natl Acad Sci U S A. 2004 Dec 21;101(51):17669-74. DOI:10.1073/pnas.0408236101 |
- Shimizu Y, Kuruma Y, Ying BW, Umekage S, and Ueda T. Cell-free translation systems for protein engineering. FEBS J. 2006 Sep;273(18):4133-40. DOI:10.1111/j.1742-4658.2006.05431.x |
- Pohorille A and Deamer D. Artificial cells: prospects for biotechnology. Trends Biotechnol. 2002 Mar;20(3):123-8. DOI:10.1016/s0167-7799(02)01909-1 |
Cell Free Extracts
- Yamane T, Ikeda Y, Nagasaka T, and Nakano H. Enhanced cell-free protein synthesis using a S30 extract from Escherichia coli grown rapidly at 42 degrees C in an amino acid enriched medium. Biotechnol Prog. 2005 Mar-Apr;21(2):608-13. DOI:10.1021/bp0400238 |
- Shimizu Y, Kuruma Y, Ying BW, Umekage S, and Ueda T. Cell-free translation systems for protein engineering. FEBS J. 2006 Sep;273(18):4133-40. DOI:10.1111/j.1742-4658.2006.05431.x |
Gene Expression
- Nomura SM, Tsumoto K, Hamada T, Akiyoshi K, Nakatani Y, and Yoshikawa K. Gene expression within cell-sized lipid vesicles. Chembiochem. 2003 Nov 7;4(11):1172-5. DOI:10.1002/cbic.200300630 |
- Ishikawa K, Sato K, Shima Y, Urabe I, and Yomo T. Expression of a cascading genetic network within liposomes. FEBS Lett. 2004 Oct 22;576(3):387-90. DOI:10.1016/j.febslet.2004.09.046 |
- Yamane T, Ikeda Y, Nagasaka T, and Nakano H. Enhanced cell-free protein synthesis using a S30 extract from Escherichia coli grown rapidly at 42 degrees C in an amino acid enriched medium. Biotechnol Prog. 2005 Mar-Apr;21(2):608-13. DOI:10.1021/bp0400238 |
- Liu T, Chen JY, Zheng Z, Wang TH, and Chen GQ. Construction of highly efficient E. coli expression systems containing low oxygen induced promoter and partition region. Appl Microbiol Biotechnol. 2005 Aug;68(3):346-54. DOI:10.1007/s00253-005-1913-6 |
- Voloshin AM and Swartz JR. Efficient and scalable method for scaling up cell free protein synthesis in batch mode. Biotechnol Bioeng. 2005 Aug 20;91(4):516-21. DOI:10.1002/bit.20528 |
- Cai L, Friedman N, and Xie XS. Stochastic protein expression in individual cells at the single molecule level. Nature. 2006 Mar 16;440(7082):358-62. DOI:10.1038/nature04599 |
Modelling
- Koslov MM and Markin VS. A theory of osmotic lysis of lipid vesicles. J Theor Biol. 1984 Jul 7;109(1):17-39. DOI:10.1016/s0022-5193(84)80108-3 |
- Idiart MA and Levin Y. Rupture of a liposomal vesicle. Phys Rev E Stat Nonlin Soft Matter Phys. 2004 Jun;69(6 Pt 1):061922. DOI:10.1103/PhysRevE.69.061922 |
- Ayton GS, McWhirter JL, McMurtry P, and Voth GA. Coupling field theory with continuum mechanics: a simulation of domain formation in giant unilamellar vesicles. Biophys J. 2005 Jun;88(6):3855-69. DOI:10.1529/biophysj.105.059436 |
- Kuyper CL, Kuo JS, Mutch SA, and Chiu DT. Proton permeation into single vesicles occurs via a sequential two-step mechanism and is heterogeneous. J Am Chem Soc. 2006 Mar 15;128(10):3233-40. DOI:10.1021/ja057349c |
- Cai L, Friedman N, and Xie XS. Stochastic protein expression in individual cells at the single molecule level. Nature. 2006 Mar 16;440(7082):358-62. DOI:10.1038/nature04599 |
- Bozic B, Kralj-Iglic V, and Svetina S. Coupling between vesicle shape and lateral distribution of mobile membrane inclusions. Phys Rev E Stat Nonlin Soft Matter Phys. 2006 Apr;73(4 Pt 1):041915. DOI:10.1103/PhysRevE.73.041915 |
Vesicle Formation Techniques
- Oberholzer T, Wick R, Luisi PL, and Biebricher CK. Enzymatic RNA replication in self-reproducing vesicles: an approach to a minimal cell. Biochem Biophys Res Commun. 1995 Feb 6;207(1):250-7. DOI:10.1006/bbrc.1995.1180 |
- Zawada ZH. Vesicles with a double bilayer. Cell Mol Biol Lett. 2004;9(4A):589-602.
- Weiss TM, Narayanan T, Wolf C, Gradzielski M, Panine P, Finet S, and Helsby WI. Dynamics of the self-assembly of unilamellar vesicles. Phys Rev Lett. 2005 Jan 28;94(3):038303. DOI:10.1103/PhysRevLett.94.038303 |
- Tamba Y and Yamazaki M. Single giant unilamellar vesicle method reveals effect of antimicrobial peptide magainin 2 on membrane permeability. Biochemistry. 2005 Dec 6;44(48):15823-33. DOI:10.1021/bi051684w |
- Zhao J, Jedlicka SS, Lannu JD, Bhunia AK, and Rickus JL. Liposome-doped nanocomposites as artificial-cell-based biosensors: detection of listeriolysin O. Biotechnol Prog. 2006 Jan-Feb;22(1):32-7. DOI:10.1021/bp050154o |
- Rogerson ML, Robinson BH, Bucak S, and Walde P. Kinetic studies of the interaction of fatty acids with phosphatidylcholine vesicles (liposomes). Colloids Surf B Biointerfaces. 2006 Mar 1;48(1):24-34. DOI:10.1016/j.colsurfb.2006.01.001 |
- Allain JM and Ben Amar M. Budding and fission of a multiphase vesicle. Eur Phys J E Soft Matter. 2006 Aug;20(4):409-20. DOI:10.1140/epje/i2006-10030-4 |
- Yin JJ, Kramer JK, Yurawecz MP, Eynard AR, Mossoba MM, and Yu L. Effects of conjugated linoleic acid (CLA) isomers on oxygen diffusion-concentration products in liposomes and phospholipid solutions. J Agric Food Chem. 2006 Sep 20;54(19):7287-93. DOI:10.1021/jf0610918 |
- Sunami T, Sato K, Matsuura T, Tsukada K, Urabe I, and Yomo T. Femtoliter compartment in liposomes for in vitro selection of proteins. Anal Biochem. 2006 Oct 1;357(1):128-36. DOI:10.1016/j.ab.2006.06.040 |
- Pautot S, Frisken BJ, and Weitz DA. Engineering asymmetric vesicles. Proc Natl Acad Sci U S A. 2003 Sep 16;100(19):10718-21. DOI:10.1073/pnas.1931005100 |
Membrane Proteins
- Kuruma Y, Nishiyama K, Shimizu Y, Müller M, and Ueda T. Development of a minimal cell-free translation system for the synthesis of presecretory and integral membrane proteins. Biotechnol Prog. 2005 Jul-Aug;21(4):1243-51. DOI:10.1021/bp049553u |
- Tamba Y and Yamazaki M. Single giant unilamellar vesicle method reveals effect of antimicrobial peptide magainin 2 on membrane permeability. Biochemistry. 2005 Dec 6;44(48):15823-33. DOI:10.1021/bi051684w |
- Bozic B, Kralj-Iglic V, and Svetina S. Coupling between vesicle shape and lateral distribution of mobile membrane inclusions. Phys Rev E Stat Nonlin Soft Matter Phys. 2006 Apr;73(4 Pt 1):041915. DOI:10.1103/PhysRevE.73.041915 |
Permeability and Diffusion
- Flaten GE, Dhanikula AB, Luthman K, and Brandl M. Drug permeability across a phospholipid vesicle based barrier: a novel approach for studying passive diffusion. Eur J Pharm Sci. 2006 Jan;27(1):80-90. DOI:10.1016/j.ejps.2005.08.007 |
- Zhao J, Jedlicka SS, Lannu JD, Bhunia AK, and Rickus JL. Liposome-doped nanocomposites as artificial-cell-based biosensors: detection of listeriolysin O. Biotechnol Prog. 2006 Jan-Feb;22(1):32-7. DOI:10.1021/bp050154o |
- Pascoe RJ, Masucci JA, and Foley JP. Investigation of vesicle electrokinetic chromatography as an in vitro assay for the estimation of intestinal permeability of pharmaceutical drug candidates. Electrophoresis. 2006 Feb;27(4):793-804. DOI:10.1002/elps.200500647 |
- Kuyper CL, Kuo JS, Mutch SA, and Chiu DT. Proton permeation into single vesicles occurs via a sequential two-step mechanism and is heterogeneous. J Am Chem Soc. 2006 Mar 15;128(10):3233-40. DOI:10.1021/ja057349c |
- Mullineaux CW, Nenninger A, Ray N, and Robinson C. Diffusion of green fluorescent protein in three cell environments in Escherichia coli. J Bacteriol. 2006 May;188(10):3442-8. DOI:10.1128/JB.188.10.3442-3448.2006 |
Stability
- Koslov MM and Markin VS. A theory of osmotic lysis of lipid vesicles. J Theor Biol. 1984 Jul 7;109(1):17-39. DOI:10.1016/s0022-5193(84)80108-3 |
- Idiart MA and Levin Y. Rupture of a liposomal vesicle. Phys Rev E Stat Nonlin Soft Matter Phys. 2004 Jun;69(6 Pt 1):061922. DOI:10.1103/PhysRevE.69.061922 |
- Riske KA and Dimova R. Electro-deformation and poration of giant vesicles viewed with high temporal resolution. Biophys J. 2005 Feb;88(2):1143-55. DOI:10.1529/biophysj.104.050310 |
- Tu ZC, Ge LQ, Li JB, and Ou-Yang ZC. Elasticity of polymer vesicles by osmotic pressure: an intermediate theory between fluid membranes and solid shells. Phys Rev E Stat Nonlin Soft Matter Phys. 2005 Aug;72(2 Pt 1):021806. DOI:10.1103/PhysRevE.72.021806 |
- Tamba Y and Yamazaki M. Single giant unilamellar vesicle method reveals effect of antimicrobial peptide magainin 2 on membrane permeability. Biochemistry. 2005 Dec 6;44(48):15823-33. DOI:10.1021/bi051684w |
- Allain JM and Ben Amar M. Budding and fission of a multiphase vesicle. Eur Phys J E Soft Matter. 2006 Aug;20(4):409-20. DOI:10.1140/epje/i2006-10030-4 |
Measurements
- Pascoe RJ, Masucci JA, and Foley JP. Investigation of vesicle electrokinetic chromatography as an in vitro assay for the estimation of intestinal permeability of pharmaceutical drug candidates. Electrophoresis. 2006 Feb;27(4):793-804. DOI:10.1002/elps.200500647 |
- Rogerson ML, Robinson BH, Bucak S, and Walde P. Kinetic studies of the interaction of fatty acids with phosphatidylcholine vesicles (liposomes). Colloids Surf B Biointerfaces. 2006 Mar 1;48(1):24-34. DOI:10.1016/j.colsurfb.2006.01.001 |
- Kuyper CL, Kuo JS, Mutch SA, and Chiu DT. Proton permeation into single vesicles occurs via a sequential two-step mechanism and is heterogeneous. J Am Chem Soc. 2006 Mar 15;128(10):3233-40. DOI:10.1021/ja057349c |
- Cai L, Friedman N, and Xie XS. Stochastic protein expression in individual cells at the single molecule level. Nature. 2006 Mar 16;440(7082):358-62. DOI:10.1038/nature04599 |
- Sunami T, Sato K, Matsuura T, Tsukada K, Urabe I, and Yomo T. Femtoliter compartment in liposomes for in vitro selection of proteins. Anal Biochem. 2006 Oct 1;357(1):128-36. DOI:10.1016/j.ab.2006.06.040 |
Applications
- Kim HJ and Jones MN. The delivery of benzyl penicillin to Staphylococcus aureus biofilms by use of liposomes. J Liposome Res. 2004;14(3-4):123-39. DOI:10.1081/lpr-200029887 |
- Zhao J, Jedlicka SS, Lannu JD, Bhunia AK, and Rickus JL. Liposome-doped nanocomposites as artificial-cell-based biosensors: detection of listeriolysin O. Biotechnol Prog. 2006 Jan-Feb;22(1):32-7. DOI:10.1021/bp050154o |
- Pellinen T, Huovinen T, and Karp M. A cell-free biosensor for the detection of transcriptional inducers using firefly luciferase as a reporter. Anal Biochem. 2004 Jul 1;330(1):52-7. DOI:10.1016/j.ab.2004.03.064 |
- Tawfik DS and Griffiths AD. Man-made cell-like compartments for molecular evolution. Nat Biotechnol. 1998 Jul;16(7):652-6. DOI:10.1038/nbt0798-652 |
Priority
- Kim HJ and Jones MN. The delivery of benzyl penicillin to Staphylococcus aureus biofilms by use of liposomes. J Liposome Res. 2004;14(3-4):123-39. DOI:10.1081/lpr-200029887 |
- Nomura SM, Tsumoto K, Hamada T, Akiyoshi K, Nakatani Y, and Yoshikawa K. Gene expression within cell-sized lipid vesicles. Chembiochem. 2003 Nov 7;4(11):1172-5. DOI:10.1002/cbic.200300630 |
- Ishikawa K, Sato K, Shima Y, Urabe I, and Yomo T. Expression of a cascading genetic network within liposomes. FEBS Lett. 2004 Oct 22;576(3):387-90. DOI:10.1016/j.febslet.2004.09.046 |
- Zhao J, Jedlicka SS, Lannu JD, Bhunia AK, and Rickus JL. Liposome-doped nanocomposites as artificial-cell-based biosensors: detection of listeriolysin O. Biotechnol Prog. 2006 Jan-Feb;22(1):32-7. DOI:10.1021/bp050154o |
- Rogerson ML, Robinson BH, Bucak S, and Walde P. Kinetic studies of the interaction of fatty acids with phosphatidylcholine vesicles (liposomes). Colloids Surf B Biointerfaces. 2006 Mar 1;48(1):24-34. DOI:10.1016/j.colsurfb.2006.01.001 |
- Cai L, Friedman N, and Xie XS. Stochastic protein expression in individual cells at the single molecule level. Nature. 2006 Mar 16;440(7082):358-62. DOI:10.1038/nature04599 |
- Sunami T, Sato K, Matsuura T, Tsukada K, Urabe I, and Yomo T. Femtoliter compartment in liposomes for in vitro selection of proteins. Anal Biochem. 2006 Oct 1;357(1):128-36. DOI:10.1016/j.ab.2006.06.040 |
- Pellinen T, Huovinen T, and Karp M. A cell-free biosensor for the detection of transcriptional inducers using firefly luciferase as a reporter. Anal Biochem. 2004 Jul 1;330(1):52-7. DOI:10.1016/j.ab.2004.03.064 |
Other Intersting Bits
- Yang P, Teo WK, and Ting YP. Design and performance study of a novel immobilized hollow fiber membrane bioreactor. Bioresour Technol. 2006 Jan;97(1):39-46. DOI:10.1016/j.biortech.2005.02.029 |
- Betts JI, Doig SD, and Baganz F. Characterization and application of a miniature 10 mL stirred-tank bioreactor, showing scale-down equivalence with a conventional 7 L reactor. Biotechnol Prog. 2006 May-Jun;22(3):681-8. DOI:10.1021/bp050369y |
- Kuruma Y, Nishiyama K, Shimizu Y, Müller M, and Ueda T. Development of a minimal cell-free translation system for the synthesis of presecretory and integral membrane proteins. Biotechnol Prog. 2005 Jul-Aug;21(4):1243-51. DOI:10.1021/bp049553u |
Suff removed from the vesicles page
The following chart cross-references the input variables to the dependent quantities. Each cell links to the protocol for that experiment. The number in each cell corresponds to the priority of that experiment.
| Lifespan | Rate of Protein Synthesis |
PoPS Effect | Membrane Traffic | |
| Temperature | 1 | 3 | ||
| Media | 2 | 4 | ||
| Parts | ||||
| Piping |
Vesicle Preparation
The protocol is based on A vesicle bioreactor as a step toward an artificial cell assembly by Vincent Noireaux and Albert Libchaber.
Materials
Equipment
- ..
- ..
- ..
- ..
Chemicals and reagents
- E.coli Extract
- A buffer that maintains pH between 7.4 and 8
- Crude extract [ribosomes (70S), tRNA, translation initiation, elongation, and termination factors]
- RNA polymerase
- 20 amino acids between 10 and 100 μM
- 4 ribonucleotides ATP, GTP, UTP, and CTP between 0.2 and 2 mM
- 8–15 mM magnesium salt
- 100–250 mM potassium salt
- An ATP regenerating system
- Sulfhydryl compounds (2-mercaptoethanol or DTT)
- Feeding Solution
- Same components as E.coli extract except the crude extract, the RNA polymerase and the kinase for the ATP regenerating system.
- To reduce osmotic pressure effect
- Dilute extract one time in feeding solution (50% extract–50% feeding)
- Supplement feeding solution with 4% extract
Supplies
- ..
- ..
- ..
- ..
Procedure
Hydration/Swelling method
Electroformation
- Use cells from Turner's project (about 10microlitres of 0.5 mg/ml each time)
- Clean electroformation cell
- Deposit lipid in chloroform onto the electrode.
- Put under vacuum for about three hours.
- Add buffer to the cell (usually contains 100mM sucrose, about 3ml)
- Pass current through the electrodes (10Hz, sinsoidal wave. Slowly increase volatage from 0 to 1V over the cause of 10 min)
- Take between 1 and 4 hours to grow.
Mineral oil method
- Dissolve egg lecithin in mineral oil at 5 mg/ml
- Heat solution at 50°C, and sonicate in a bath
- Incubate overnight at room temperature
- Collect 10–20 μl of the clear supernatant in a tube
- In another tube, add 1 μl of the E.coli cell extract to 200 μl of mineral oil with dissolved phospholipids
- Gently vortex for a few seconds to obtain an extract-oil emulsion
- Place 50 μl of the emulsion on top of the feeding solution to form a monolayer of phospholipids at the interphase
- Centrifuge to form vesicles of 1 to a few tens of μm in diameter
- Recover the vesicles in the feeding solution
Total Time Required
Quantities Varied
Notes
- Reaction to be carried out at room temperature (25°C)
- Use BSA–rhodamine isothiocyanate and fluorescein-12-UTP to test vesicle leakage
- Selective permeability of the vesicles is obtained with the α-hemolysin toxin from S. aureus
List of Experiments
Lifespan as a function of Temperature
Materials
Equipment
- ..
- ..
- ..
- ..
Chemicals and reagents
- ..
- ..
- ..
- ..
Supplies
- ..
- ..
- ..
- ..
Procedure
- Place 10 μl of in vitro expression system in 7 tubes
- Incubate each tube in 4, 10, 15, 20, 25, 30, or 37°C
- Measure GFP expression using photomultiplier tube at one hour intervals up to 5h
- Plot a graph of GFP expression against time at each temperature
Total Time Required
Quantities Varied
Notes
Lifespan as a function of Media
Materials
Equipment
- ..
- ..
- ..
- ..
Chemicals and reagents
- ..
- ..
- ..
- ..
Supplies
- ..
- ..
- ..
- ..
Procedure
- ..
- ..
- ..
- ..
Total Time Required
Quantities Varied
Notes
Protein Synthesis as a function of Temperature
Materials
Equipment
- ..
- ..
- ..
- ..
Chemicals and reagents
- ..
- ..
- ..
- ..
Supplies
- ..
- ..
- ..
- ..
Procedure
- ..
- ..
- ..
- ..
Total Time Required
Quantities Varied
Notes
Protein Synthesis as a function of Media
Materials
Equipment
- ..
- ..
- ..
- ..
Chemicals and reagents
- ..
- ..
- ..
- ..
Supplies
- ..
- ..
- ..
- ..
Procedure
- ..
- ..
- ..
- ..
Total Time Required
Quantities Varied
Notes
Experiments
Testing In Vitro system
1. Do both S30 and S12 extract on the K12 strain at 25°C.
2. Add E.coli and T7 RNA polymerases separately to each cell extract.
3. Make a reporter gene construct for GFP using pLux and T7 promoters respectively.
4. Mix gene contructs with S30 cell extracts.
*E.coli RNAP + pLux
*T7 RNAP + T7 promoter
5. Repeat step 4 with S12 cell extracts.
6. Measure fluorescence intensity at hourly intervals for 5 hours.
7. Determine the optimal translational activity of S30 and S12.
8. Choose which ever is best for strain K12.
9. Carry out subsequent experiments with the appropriate cell extract.
Testing Temperature Dependence
1. Carry out steps 2 to 6 at 4°C, 10°C, 15°C, 20°C, 25°C, 30°C, and 37°C; at pH 7.
2. Measure fluorescence intensity at hourly intervals for 5 hours.
Testing pH Dependence
1. Carry out steps 2 to 6 at pH 2, 4, 6, 7, 8, 10, 12; at 25°C.
2. Measure fluorescence intensity at hourly intervals for 5 hours.
Testing Lifespan of the system
Testing In Veso System
1. Follow the protocol for vesicle preparation in the "Design" section.
2. Repeat steps 2 to 6 above using only the optimal cell extract solution.
Testing for membrane trafficking and pore formation
1. Insert fluorescent markers BSA-rhodamine and UTP-fluorescein into the cell extract before vesicle formation.
2. Follow protocol for vesicle formation.
3. Observe fluorescence patterns in the feeding solution and vesicles.
Testing Temperature Dependence
1. Carry out steps 2 to 6 at 4°C, 10°C, 15°C, 20°C, 25°C, 30°C, and 37°C; at pH 7.
2. Measure fluorescence intensity at hourly intervals for 5 hours.
Testing pH Dependence
1. Carry out steps 2 to 6 at pH 2, 4, 6, 7, 8, 10, 12; at 25°C.
2. Measure fluorescence intensity at hourly intervals for 5 hours.
Testing Lifespan of the system
DNA Constructs
Generic Constructs
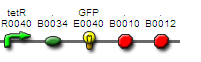
pTet GFP
- Registry part: BBa_I13522
- Comments: Untagged GFP behind a constitutive promoter.
- DNA Available in the registry
- for use in CBD
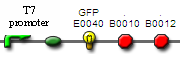
T7 promoter
- Registry part: BBa_J34814
- T7 promoter sequence: gaatttaatacgactcactatagggaga
- Comments: T7 Promoter to be used qith t7 polymerase BBa_J34811
- DNA still in planning
- for use in CBD
- pT7 sequence from Ambion:
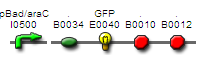
Arabinose -> pBad = GFP
- Registry part: BBa_J5528
- Comments: Similar to part J5527 where mRFP is replaced with GFP
- DNA Available in the registry
- for use in CBD
The Biofilm Saboteur
Summary
Biofilms are a huge problem in medicine and in industry. The dispersal of biofilms has long posed a problem to biologists, chemists, and engineers. Recently, a paper[11] was published describing the use of an engineered bacteriophage that would also produce DspB, an enzyme that breaks down one of the major structural components of biofilm. However, it was pointed out that bacteriophages target very specific cells, which may not be present in the biofilm under consideration. Furthermore, bacteria often evolve defences against phages. Thus, although the phage technique has proven that the principle of infecting a biofilm is a good strategy to disperse it, it is far from becoming usable outside the tightly controlled lab environments.
This project proposes an alternative method of biofilm dispersal. In short, a bacteria will be modified to express DspB as a response to three input signals - quorum sensing, biofilm presence, and anaerobic conditions. These bacteria can then be sprayed over a biofilm, which they infiltrate, and disperse.
There are three major assumptions that may prove problematic in this proposal:
- That the saboteur bacteria will be able to penetrate the biofilm
- That the saboteur bacteria will survive in the biofilm
- That the quorum sensing mechanism will work
A list of references can be found at the bottom, that may be able to help addressing these concerns.
The Hrp system may be used with its three inputs: biofilm detection, quorum sensing, and anaerobic conditions sensing (inverted in V input). By inverting the anaerobic conditions sensing, a basal level (the 10% leakage of the V-inhibition) of DspB will be produced. As soon as anaerobic conditions are reached, V-expression is inhibited (the inversion) which kicks the Hrp system into full swing.
Resources
Imperial College contacts:
Web pages:
- Biofilms Online
- Biofilm Hypertextbook
- With a very rich references section.
Biofilms and your health...
- Biofilms are responsible for diseases such as otitis media, the most common acute ear infection in children in the U.S. Other diseases in which biofilms play a role include bacterial endocarditis (infection of the inner surface of the heart and its valves), cystic fibrosis (a chronic disorder resulting in increased susceptibility to serious lung infections), and Legionnaire's disease (an acute respiratory infection resulting from the aspiration of clumps of Legionnella biofilms detached from air and water heating/cooling and distribution systems).
- Biofilms may be responsible for a wide variety of nosocomial (hospital-acquired) infections. Sources of biofilm-related infections can include the surfaces of catheters, medical implants, wound dressings, or other types of medical devices.
- Biofilms are highly resistant to antibiotics. Consequently, very high and/or long-term doses are often required to eradicate biofilm-related infections.
- Biofilms happily colonize many household surfaces, including toilets, sinks, countertops, and cutting boards in the kitchen and bath. Poor disinfection practices and ineffective cleaning products may increase the incidence of illnesses associated with pathogenic organisms associated with normal household activity.
Biofilms and industry...
- Biofilms are responsible for billions of dollars in lost industrial productivity and both product and capital equipment damage each year. For example, biofilms are notorious for causing pipe plugging, corrosion and water contamination.
- Biofilm contamination and fouling occurs in nearly every industrial water-based process, including water treatment and distribution, pulp and paper manufacturing, and the operation of cooling towers.
From Biofilms Online
Notes
The metabolism of cells is different at different depths within the biofilm. At the deepest level of a mature biofilm, anaerobic respiration may predominate.
Clusters of nitrite oxidizers crowd around distinct clusters of ammonia oxidizers (20, 29) (see references above). Thus, is the metabolic waste product of the ammonia oxidizers, nitrite, made available to the bacteria that can use it as a substrate for oxidation. The activities of these commingled species lead to the consumption of ammonia and oxygen near the biofilm surface and the simultaneous production and consumption of nitrite slightly below the biofilm surface.
Biofilm References
- Xu KD, Stewart PS, Xia F, Huang CT, and McFeters GA. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl Environ Microbiol. 1998 Oct;64(10):4035-9. DOI:10.1128/AEM.64.10.4035-4039.1998 |
- Xu KD, Franklin MJ, Park CH, McFeters GA, and Stewart PS. Gene expression and protein levels of the stationary phase sigma factor, RpoS, in continuously-fed Pseudomonas aeruginosa biofilms. FEMS Microbiol Lett. 2001 May 15;199(1):67-71. DOI:10.1111/j.1574-6968.2001.tb10652.x |
- Wood BD, Quintard M, and Whitaker S. Calculation of effective diffusivities for biofilms and tissues. Biotechnol Bioeng. 2002 Mar 5;77(5):495-516. DOI:10.1002/bit.10075 |
- Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, and Greenberg EP. Gene expression in Pseudomonas aeruginosa biofilms. Nature. 2001 Oct 25;413(6858):860-4. DOI:10.1038/35101627 |
- Stoodley P, Debeer D, and Lewandowski Z. Liquid flow in biofilm systems. Appl Environ Microbiol. 1994 Aug;60(8):2711-6. DOI:10.1128/aem.60.8.2711-2716.1994 |
- Stewart PS. A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms. Biotechnol Bioeng. 1998 Aug 5;59(3):261-72. DOI:10.1002/(sici)1097-0290(19980805)59:3<261::aid-bit1>3.0.co;2-9 |
- Sternberg C, Christensen BB, Johansen T, Toftgaard Nielsen A, Andersen JB, Givskov M, and Molin S. Distribution of bacterial growth activity in flow-chamber biofilms. Appl Environ Microbiol. 1999 Sep;65(9):4108-17. DOI:10.1128/AEM.65.9.4108-4117.1999 |
- Redfield RJ. Is quorum sensing a side effect of diffusion sensing?. Trends Microbiol. 2002 Aug;10(8):365-70. DOI:10.1016/s0966-842x(02)02400-9 |
- Picioreanu C, van Loosdrecht MC, and Heijnen JJ. Mathematical modeling of biofilm structure with a hybrid differential-discrete cellular automaton approach. Biotechnol Bioeng. 1998 Apr 5;58(1):101-16. DOI:10.1002/(sici)1097-0290(19980405)58:1<101::aid-bit11>3.0.co;2-m |
- O'Toole GA, Gibbs KA, Hager PW, Phibbs PV Jr, and Kolter R. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J Bacteriol. 2000 Jan;182(2):425-31. DOI:10.1128/JB.182.2.425-431.2000 |
- Lu TK and Collins JJ. Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci U S A. 2007 Jul 3;104(27):11197-202. DOI:10.1073/pnas.0704624104 |
- MacLeod FA, Guiot SR, and Costerton JW. Layered structure of bacterial aggregates produced in an upflow anaerobic sludge bed and filter reactor. Appl Environ Microbiol. 1990 Jun;56(6):1598-607. DOI:10.1128/aem.56.6.1598-1607.1990 |
- Kreft JU, Picioreanu C, Wimpenny JW, and van Loosdrecht MC. Individual-based modelling of biofilms. Microbiology (Reading). 2001 Nov;147(Pt 11):2897-912. DOI:10.1099/00221287-147-11-2897 |
- Jackson DW, Suzuki K, Oakford L, Simecka JW, Hart ME, and Romeo T. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J Bacteriol. 2002 Jan;184(1):290-301. DOI:10.1128/JB.184.1.290-301.2002 |
- Hermanowicz SW. A simple 2D biofilm model yields a variety of morphological features. Math Biosci. 2001 Jan;169(1):1-14. DOI:10.1016/s0025-5564(00)00049-3 |
- Harmsen HJ, Kengen HM, Akkermans AD, Stams AJ, and de Vos WM. Detection and localization of syntrophic propionate-oxidizing bacteria in granular sludge by in situ hybridization using 16S rRNA-based oligonucleotide probes. Appl Environ Microbiol. 1996 May;62(5):1656-63. DOI:10.1128/aem.62.5.1656-1663.1996 |
- Dockery JD and Keener JP. A mathematical model for quorum sensing in Pseudomonas aeruginosa. Bull Math Biol. 2001 Jan;63(1):95-116. DOI:10.1006/bulm.2000.0205 |
- Costerton JW, Lewandowski Z, DeBeer D, Caldwell D, Korber D, and James G. Biofilms, the customized microniche. J Bacteriol. 1994 Apr;176(8):2137-42. DOI:10.1128/jb.176.8.2137-2142.1994 |
- Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, and Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998 Apr 10;280(5361):295-8. DOI:10.1126/science.280.5361.295 |
Favourite Ideas
- Detecting, collecting, and packaging of 'garbage'
- Solar powered bacteria
- characterisation of HRP part
- UV sensitivity in the SOS gene initiating apoptosis (instead of DNA repair)
- Use of HRP AND gate function: substrate present AND quorum, start collection. Third signal for full cell stops collection, and starts fluorescence.
Step 1: detection using biosensor leads to cell replication Step 2: as cells divide, quorum is reached Step 3: collection apparatus is built, and collection begins Step 4: the cell fills up, and is not able to collect anymore Step 5: the cell signals itself to stop collection, and begin fluorescence
Method for trapping the cells in a matrix in order to pass water through. What rate can water be passed through?
(maybe the HRP part can be used in the collector system above?)
What we should do regardless of choice:
- in-vitro parts testing
- chassis characterisation
HRP System Notes
- When controlling R & S, use promoters with equal rates
- HrpBOX is VERY specific
- 50 sigma54 systems in e.coli, and about 20 sigma54 regulators
- use Suhail to find S and V interaction
- Lon proteins degrade R
- The stability (half-life) of R & S is not characterised
- The response time is not characterised, but it is very fast
- How strongly does V bind S? Are there any interfering factors?
Protocols
- Three 5 ml cultures of supplemented M9 medium and antibiotic (kanamycin, 20 µg/ml) were inoculated with single colonies from a freshly streaked plate of MG1655 containing <partinfo>BBa_T9002</partinfo>. One 5 ml culture was inoculated with a single colony from a freshly streaked plate of MG1655 containing a <partinfo>BBa_T9002</partinfo> mutant lacking a GFP expression device (see the stability section).
- Cultures were grown in 17 mm test tubes for 15 hrs at 37°C with shaking at 70 rpm.
- Cultures were diluted 1:1000 into 5.5 ml of fresh medium and grown to an OD600 of 0.15 under the same conditions as before. This growth took on average 4.25 hrs.
- Twenty-four 200 µl aliquots of each of the cultures were transferred into a flat-bottomed 96 well plate (Cellstar Uclear bottom, Greiner).
- 2 µl of the stock concentrations of the cognate AHL, 3-oxohexanoyl-homoserine lactone (3OC6HSL), was added to each well to yield 8 different final concentrations (0, 1E-10, 1E-9, 1E-8, 1E-7, 1E-6, 1E-5 and 1E-4 M). Three replicate wells were measured for each concentration of 3OC6HSL. Three wells were each filled with 200 µl of medium to measure the absorbance background. Three further wells were each filled with 200 µl of the <partinfo>BBa_T9002</partinfo> mutant culture to measure fluorescent background.
- The plate was incubated in a Wallac Victor3 multi-well fluorimeter (Perkin Elmer) at 37°C and assayed with an automatically repeating protocol of absorbance measurements (600 nm absorbance filter, 0.1 second counting time through 5 mm of fluid), fluorescence measurements (488 nm excitation filter, 525 nm emission filter, 0.5 seconds, CW lamp energy 12901 units), and shaking (1 mm, linear, normal speed, 5 seconds). Time between repeated measurements was 2 min and 21 s. Approximately 6 min elapsed between beginning addition of 3OC6HSL to the wells and the first plate reader measurement. 3OC6HSL was added in order of increasing concentration to minimize GFP synthesis during plate loading. Cells appear to grow exponentially for the duration of the plate reader measurement protocol (see Figure 2 for representative growth curves).
- We repeated steps 1 through 6 on three separate days to obtain data for nine colonies from a single plate.
- Data processing was used to calculate the GFP synthesis rate for each well and is described on the Data analysis page. The data for each colony tested was averaged across the three replicate wells. The mean for each colony was then averaged to obtain a global weighted mean. The contribution of each data point to the mean was weighted in inverse proportion to the square root of the uncertainty in the data point. The time-dependent input-output surface is shown above in Figure 3. Following an initial transient response, device output reached an approximate steady state.
- The snapshot transfer function in Figure 1 is the 60 min time-slice from the surface shown in Figure 3 (highlighted as a heavy black line). Error bars in Figure 1 representing the 95% confidence interval for the global weighted mean are smaller than the data markers. At this time point, the output of the device ranges from a minimum of 0 to a maximum of 503 molecules GFP CFU-1 s-1. Characteristics of the snapshot transfer curve were defined relative to that maximum output.
- The Input Swing is defined as the minimum range of measured inputs for which device output ranges from 5% to 95% of its maximum value.
- The Switch Point is defined as the input level at which device output is 50% of the maximum output. This characteristic is calculated by linear interpolation between the measured data points. The uncertainty in the switch point was calculated by propagating the uncertainty in the measurement of the maximum output level through the switch point calculation.
- Two cultures, one of MG1655 bearing <partinfo>BBa_T9002</partinfo> and one of MG1655 bearing the <partinfo>BBa_T9002</partinfo> mutant lacking a GFP expression device (see stability section) were prepared as described in steps 1 – 3 of the transfer function protocol.
- Six 200 µl aliquots of the <partinfo>BBa_T9002</partinfo> culture were transferred into a flat-bottom 96 well plate.
- Three wells were each filled with 200 μl of medium to measure the absorbance background. Three further wells were each filled with 200 μl of the <partinfo>BBa_T9002</partinfo> mutant culture to measure fluorescent background. 3OC6HSL was added to three of the wells containing the <partinfo>BBa_T9002</partinfo> culture to a final concentration of 1E-7 M.
- The plate was incubated in a Wallac Victor3 multi-well fluorimeter at 37°C and assayed with an automatically repeating protocol of fluorescence measurements, absorbance measurements, and shaking (all as described in the transfer function protocol). Time between repeated measurements was 54 s.
- Data processing is described on the Data analysis page. The short interval between measurements resulted in apparent noise in the calculated GFP synthesis rates. Thus, the time response data shown in Figure 1 had an additional processing step in which the raw fluorescence data was smoothed using MATLAB’s, rlowess smoothing filter across five time points. The same data, processed without the smoothing filter, is shown in Figure 2. In both Figure 1 and Figure 2, the error bars represent the 95% confidence interval for the mean.
- The low-to-high (LH) response time of the device was parameterized by calculating the time taken for the output to rise to 50% and 90% of its maximum value. These times were calculated by linearly interpolating between the measured data values.
- Two cultures, one MG1655 bearing <partinfo>BBa_T9002</partinfo> and one of MG1655 bearing the <partinfo>BBa_T9002</partinfo> mutant lacking a GFP expression device (see stability section) were prepared as described in steps 1 – 3 of the transfer function section above. However, in this case the overnight cultures were diluted into 20 ml of fresh medium in a 200 ml flask and shaken at 220 rpm during growth.
- Three of the eight AHL variants (see table below) were preloaded into a flat-bottom 96 well plate to eight different final concentrations (0, 1E-10, 1E-9, 1E-8, 1E-7, 1E-6, 1E-5 M, and 1E-4 M). Three wells were each filled with 200 μl of media to measure the absorbance background. Three further wells were filled with 200 µl of the mutant <partinfo>BBa_T9002</partinfo> culture to measure the fluorescent background.
- Seventy-two 200 µl aliquots of the <partinfo>BBa_T9002</partinfo> culture were transferred to the plate. Three replicate wells were filled for each concentration of each AHL.
- The plate was incubated in a Wallac Victor3 multi-well fluorimeter at 37°C and assayed with an automatically repeating protocol of absorbance measurements, fluorescence readings, and shaking (as described in the transfer function protocol). Time between repeated measurements was 2 min and 21 s.
- Steps 1 through 4 were repeated once with three more of the AHL variants and again with the final two AHL variants. The time between repeated measurements was kept fixed in each case.
- Data processing is described here. In Figure 1, snapshot transfer functions are plotted for each AHL variant at the 60 min time point similar to the transfer function experiment. The error bars represent the 95% confidence interval for the mean of the three replicate wells of each measurement.
| Full Name | Molecule abbreviation | Species | Notes | Source | Images (from Sigma Aldrich) |
|---|---|---|---|---|---|
| Butanoyl-homoserine lactone | C4HSL | P. aeruginosa | Sigma Aldrich (#09945) | File:Butryl-homoserine lactone.GIF | |
| 3-oxohexanoyl-homoserine lactone | 3OC6HSL | V. fischeri | Lux system signaling molecule | Sigma Aldrich (#K3007) | File:3-oxohexanoyl-homoserine lactone.GIF |
| Hexanoyl-homoserine lactone | C6HSL | C. violaceum | Very similar to 3OC6HSL | Sigma Aldrich (#09926) | File:Hexanoyl-homoserine lactone.GIF |
| Heptanoyl-homoserine lactone | C7HSL | E. psidii R. IBSBF 435T | Sigma Aldrich (#10939) | File:Heptanoyl-homoserine lactone.GIF | |
| Octanoyl-homoserine lactone | C8HSL | B. cepacia, V. fischeri | Sigma Aldrich (#10940) | File:Octanoyl-homoserine lactone.GIF | |
| 3-oxoctanoyl-homoserine lactone | 3OC8HSL | A. tumefaciens | Sigma Aldrich (#O1764) | File:3-Oxooctanoyl-L-homoserine lactone2.GIF | |
| Decanoyl-homoserine lactone | C10HSL | B. pseudomallei | Sigma Aldrich (#17248) | File:Decanoyl-homoserine lactone.GIF | |
| Dodecanoyl-homoserine lactone | C12HSL | Synthetic | Sigma Aldrich (#17247) | File:Dodecanoyl-homoserine lactone.GIF |
- A 5 ml culture of supplemented M9 medium and antibiotic (kanamycin, 20 µg/ml) was inoculated with a single colony of <partinfo>BBa_T9002</partinfo> from a fresh plate.
- The culture was grown in a 17 mm test tube for 15 hrs at 37°C with shaking at 70 rpm.
- The culture was diluted 1:400 into 5 ml of fresh medium and grown under identical conditions for a further 10 hrs. The culture was diluted 1:4096 into two identical 5 ml cultures. 3OC6HSL was added to one of the cultures to an input level of 1E-7 M.
- These two cultures were propagated in a similar manner with 1:400 and 1:4096 dilutions in the morning and evening respectively every day for 5 days.
- Each day, following the overnight incubation of cultures, a sample of the overnight cultures was stored in a 20% glycerol solution at –80°C to allow later sequencing. Samples to be sequenced were streaked on LB plates containing kanamycin (20 µg/ml). Plasmid DNA from five colonies on each plate was purified using a Qiagen Spin Miniprep Kit (Qiagen). DNA was sequenced and analyzed as described earlier using standard primers internal and external to the receiver and reporter device (BBa_G00100, BBa_G00101, and G00602).
- Each morning, a second culture was inoculated by a 1:400 dilution from the overnight culture for both the high and low input condition. These second cultures were grown in the absence of 3OC6HSL for 8 hrs. This growth period diluted-out the accumulated GFP from the culture propagated in the presence of 3OC6HSL before assaying performance.
- Samples from both of the second copies were induced with a high input level of 3OC6HSL (1E-7 M) at 37°C with shaking at 70 rpm for 45 min. Single-cell fluorescence measurements were carried out on a FACScan flow cytometer (Becton-Dickinson) with a 488 nm Argon excitation laser and 525 nm emission filter. During each flow-cytometer measurement, data was collected from 50,000 cells. 2 µl of Sphero fluorescent beads (0.87 µm ø, Spherotech) in 500 µl H2O was used as a control for experiment-to-experiment variation of cytometer performance. FACScan data were analyzed using Cell Quest (Becton-Dickinson) and FlowJo (FlowJo).
- Genetic stability is defined as the number of replication events until a mutant becomes fixed in the population. The performance stability of a device is defined as the number of replication events until the majority of the devices in the population have lost the ability to respond correctly to an input. Both the genetic and the performance stability were estimated from the FACS data shown in Figure 1 and genetic stability was confirmed by sequence analysis.
Feasibility Criteria
Level 1
- Application driven
- Sources of knowledge / research
- reuse old knowledge
- novel, no overlap with other iGEMs
- project must be approves (Ethics, Safety)
- scope - needs to be functional in 9 weeks
Level 2
- Application has to be low cost
- has to be portable
- safe, no contamination
- inspire confidence in synthetic biology
- must have a contingency
- use parts in registry
- no heavy metals
- no human testing
- no quantum shift (big change)
- keep to the status quo
- base on a commercialised product
- budget
- equipment
Level 3
- based on reliable, basic parts
- max 3 components
- well documented
- easily modelled
- limited to college grounds
- restricted booking
- easy learning curve
Level 4
- well characterised biobricks and chassis
- use PoPS
Tree Structure
- Application driven
- Application has to be low cost
- has to be portable
- safe, no contamination
- inspire confidence in synthetic biology
- must have a contingency
- Sources of knowledge / research
- Reuse old knowledge
- use parts in registry
- based on reliable, basic parts
- well characterised biobricks and chassis
- avoid composite systems
- standards adherence
- use PoPS
- based on reliable, basic parts
- use parts in registry
- Novel and no overlap with past iGEMS
- Project must be approved, Safety & Ethics
- no heavy metals
- no human testing
- Scope - needs to be functional in 9 weeks
- no quantum shift (big change)
- max 3 components
- well documented
- easily modelled
- keep to the status quo
- base on a commercialised product
- budget
- equipment
- limited to college grounds
- restricted booking
- easy learning curve
- no quantum shift (big change)