User:Cristhian Carrillo/Notebook/Physics 307L/2010/10/13
From OpenWetWare
Jump to navigationJump to search
Balmer Series
- Note that Ginny was my lab partner for this lab.
Purpose
- Observe the Balmer Series of Hydrogen and Deuterium.
- The purpose of this lab is to study the Balmer Series in the Hydrogen spectrum.
- Determine the Rydberg constant for Hydrogen.
- Compare Hydrogen with Deuterium.
- Learning how to calibrate an optical spectrometer using the known mercury spectrum.
Equipment
- Constant Deviation Spectrometer
- Spectrum Tube Power Supply Model SP200, 5000 volts, 10MA.
- Mercury and Hydrogen tubes
Safety
- Be careful with the equipment.
- Check to make sure that the electrical wires have no electrocution points.
- Be careful with the glass tubes.
- One very important thing is to turn the screw that rotates the prism in one direction only to avoid gear back lash
Setup
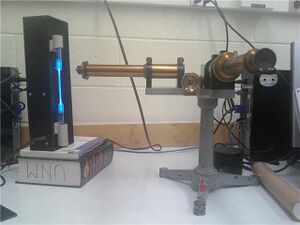

- We followed Professor Gold's Manual for the setup.
Calibration
- Turn on the mercury tube and let it warm up for a few minutes
- Find a line of the mercury spectrum with the spectrometer slit wide (1/2 to 1mm).
- The narrower the slit, the better
- Keep in mind that narrowing the slit causes loss of intensity of the light.
- Locate all mercury lines that you can.
- When you are turning the screw which rotates the prism, note the positionis on the dial which correspond to the mercury lines.
Steps to begin the experiment
- Must bring the slit into focus by turning the large ring near the center of the viewing microscope.
- Attach and position the mercury bulb into the spectrum tube power supply.
- After turning on the power supply, allow the mercury bulb to warm up for about five minutes.
- When calibrating the spectrometer, use a wide slit setting to find a line of the mercury spectrum and narrow the slit until the line comes into a sharp focus.
- You must locate all mercury spectra lines and note the position or the value of your spectrometer dial.
- Use the known values of the light wavelengths to finish calibrating the system.
- Use the data to find the correct quantum numbers corresponding to the wavelengths.
- Use the equation to solve for Rydberg's constant R in each case.
- Repeat this process for deuterium.
Calculations and Analysis
Error in widget Google Spreadsheet: Unable to load template 'wiki:Google Spreadsheet'
- After we calibrated with mercury we measured the wavelengths in hydrogen and deuterium by reading the dial
- For my calculations I used the second data set for hydrogen and deuterium since I thought that we took more than enough measurements.
Equations Used for Calculations
- For Hydrogen
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{2^2}-\frac{1}{n^2}), n=3,4,5,..\,\! }[/math]
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{2^2}-\frac{1}{n^2}), n=3,4,5,..\,\! }[/math]
- The general equation:
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{m^2}-\frac{1}{n^2}) }[/math]
- [math]\displaystyle{ m=1,2,3,...\,\! }[/math]
- [math]\displaystyle{ n=2,3,4,5,...\,\! }[/math]
- [math]\displaystyle{ n\gt m\,\! }[/math]
- [math]\displaystyle{ m=1,2,3,...\,\! }[/math]
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{m^2}-\frac{1}{n^2}) }[/math]
- We calculated the accepted value of Rydberg's constant from the following equation found on Professor Gold's Manual:
- [math]\displaystyle{ R=\frac{\mu e^4}{8\epsilon _0^2ch^3}\,\! }[/math]
- Where [math]\displaystyle{ \mu\,\! }[/math] is the reduced mass
- [math]\displaystyle{ R=1.0967758\times 10^7 m^{-1}\,\! }[/math]
- The following accepted values for the four visible wavelengths of the Balmer Series were taken from the hyperphysics website
- [math]\displaystyle{ n=6\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda =410.174 nm\,\! }[/math]
- [math]\displaystyle{ n=5\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda =434.047 nm\,\! }[/math]
- [math]\displaystyle{ n=4\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda =486.133 nm\,\! }[/math]
- [math]\displaystyle{ n=3\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda =656.272 nm\,\! }[/math]
- [math]\displaystyle{ n=6\rightarrow n=2\,\! }[/math]
- Using the results from the data set 2 I calculated the values for the wavelengths. Please follow this link File:Balmer calculations.xlsx to see the standard deviation and standard error of the mean for our data. The values below are what I calculated in the excel spread sheet.
- [math]\displaystyle{ n=6\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda_{Hydrogen} =417.88 nm\,\! }[/math]
- [math]\displaystyle{ \lambda_{Deuterium} =N/A\,\! }[/math]
- [math]\displaystyle{ n=5\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda_{Hydrogen} =433.66 nm\,\! }[/math]
- [math]\displaystyle{ \lambda_{Deuterium} =433.7 nm\,\! }[/math]
- [math]\displaystyle{ n=4\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda_{Hydrogen} =483.44 nm\,\! }[/math]
- [math]\displaystyle{ \lambda_{Deuterium} =483.17 nm\,\! }[/math]
- [math]\displaystyle{ n=3\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda_{Hydrogen} =644.19 nm\,\! }[/math]
- [math]\displaystyle{ \lambda_{Deuterium} =642.07 nm\,\! }[/math]
- [math]\displaystyle{ n=6\rightarrow n=2\,\! }[/math]
- Using these values,I was able to calculate our measured Rydberg's constant.
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{2^2}-\frac{1}{n^2}), n=3,4,5,6\,\! }[/math]
- [math]\displaystyle{ R=\frac{4n^2}{\lambda(n^2-4)}\,\! }[/math]
- [math]\displaystyle{ R=\frac{4n^2}{\lambda(n^2-4)}\,\! }[/math]
- [math]\displaystyle{ n=6\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ R_{Hydrogen}=\frac{4(6)^2}{(417.88\times10^{-9} m)((6)^2-4)}\approx1.0768641\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ R_{Hydrogen}=\frac{4(6)^2}{(417.88\times10^{-9} m)((6)^2-4)}\approx1.0768641\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ n=6\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ n=5\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ R_{Hydrogen}=\frac{4(5)^2}{(433.66\times10^{-9} m)((5)^2-4)}\approx1.0980733\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ R_{Deuterium}=\frac{4(5)^2}{(433.7\times10^{-9} m)((5)^2-4)}\approx1.0979720\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ R_{Hydrogen}=\frac{4(5)^2}{(433.66\times10^{-9} m)((5)^2-4)}\approx1.0980733\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ n=5\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ n=4\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ Ra_{Hydrogen}=\frac{4(4)^2}{(483.44\times10^{-9} m)((4)^2-4)}\approx1.1032048\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ R_{Deuterium}=\frac{4(4)^2}{(483.17\times10^{-9} m)((4)^2-4)}\approx1.1038212\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ Ra_{Hydrogen}=\frac{4(4)^2}{(483.44\times10^{-9} m)((4)^2-4)}\approx1.1032048\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ n=4\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ n=3\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ R_{Hydrogen}=\frac{4(3)^2}{(644.19\times10^{-9} m)((3)^2-4)}\approx1.1176826\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ R_{Deuterium}=\frac{4(3)^2}{(642.07\times10^{-9} m)((3)^2-4)}\approx1.1213730\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ R_{Hydrogen}=\frac{4(3)^2}{(644.19\times10^{-9} m)((3)^2-4)}\approx1.1176826\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ n=3\rightarrow n=2\,\! }[/math]
- Below are the average values of the Rydberg constant for Hydrogen and Deuterium.
- [math]\displaystyle{ R_{Hydrogen,average}\approx1.0989562\pm 0.008\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ R_{Deuterium,average}\approx1.1077221\pm 0.007\times10^7 m^{-1}\,\! }[/math]
- Below are the calculated percent errors.
- [math]\displaystyle{ \% error=\frac{R_{accepted}-R_{measured}}{R_{accepted}} }[/math]
- [math]\displaystyle{ \% error_{Hydrogen}\approx0.20%\,\! }[/math]
- [math]\displaystyle{ \% error_{Deuterium}\approx.998%\,\! }[/math]
Error
- Reasons for our error could be because...
- We might have calibrated the spectroscope wrong
- Some line spectra were hard to see
- Gear backlash: We might have turned the dial in both directions