User:Colette M McConnell/Notebook/Biology 210 at AU
March 21, 2014 Embryology Lab
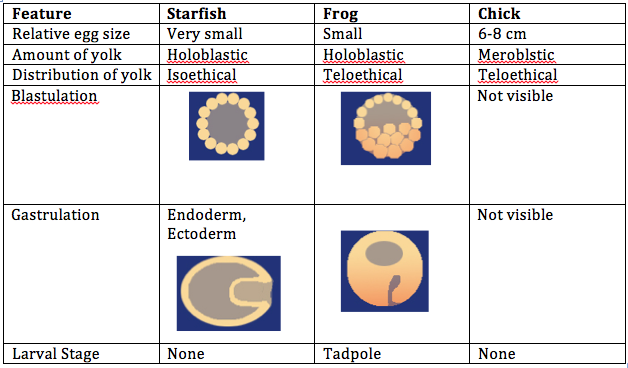
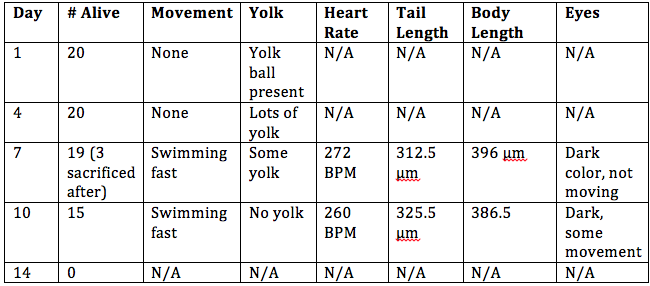
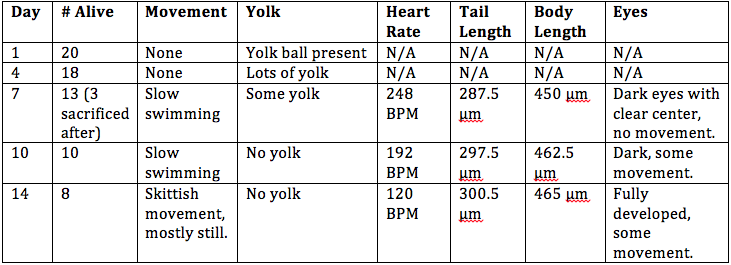
Introduction: The objective of this lab was to observe the stages of embryonic development in different organisms and create an experiment to study how various environmental conditions affect embryonic development in zebrafish. We compared the embryonic stages of a starfish, frog, and chick. For the zebrafish experiment, the condition tested was observing embryonic development in a dark environment.
Procedures: 1. Examine prepared slides of starfish and frog embryos under a microscope. Observe the size, amount and distribution of yolk, blastulation, gastrulation, and larval stage. 2. Examine living 72 hour chick embryos under a dissecting scope. Observe the size, amount and distribution of yolk, blastulation, gastrulation, and larval stage. 3. Choose an experimental condition to test for the zebrafish experiment. 4. Observe zebrafish embryos and determine their developmental stage. 5. Set up a control group petri dish and a variable group control dish. Add 20 mls of deerpark water and 20 healthy embryos to each dish. 6. When embryos are 4-5 days old, remove 10 mls of water and add 25 mls of fresh water. 7. Observe a representative embryo from each dish on a depression slide and observe under a microscope. Observe degree of body and tail pigmentation, eyes and eye movement, heartrate, pectoral fin development, yolk sac size, development of swim bladder, development of mouth, and general movement. 8. At 7 days old, remove 5 mls of water with any dead embryos and add 5 mls of fresh water. Preserve 1 to 3 embryos from each dish in the paraformaldehyde. 9. Repeat step 7. 10. Between week 1 and week 2, remove 5 mls of water and add 10 mls. 11. Repeat step 7. 12. Feed 2 drops of paramecium after 1 week. 13. Make final observations and measurements after 2 weeks (step 7, as well as measure the length of the entire larvae and measure the diameter of the eyes).
Embryo Observations:
Control group:
Conclusion: The objective of this lab to study the embryonic stages of a starfish, frog, and chick were met, as well as creating a successful experiment by testing an independent variable on the effect of embryonic development in zebrafish. It was concluded that zebrafish develop better in a dark environment, however they swim slower and are generally smaller than zebrafish in the control group. There was an experimental error in this experiment; it is believed that all of the zebrafish in the control group died before observing them at 2 weeks because the room temperature was too warm for them to survive. In the future, this experiment would be more successful if the control and variable groups were kept at the same temperature that is slightly cooler than room temperature. CM
March 12, 2014 Mini Lab
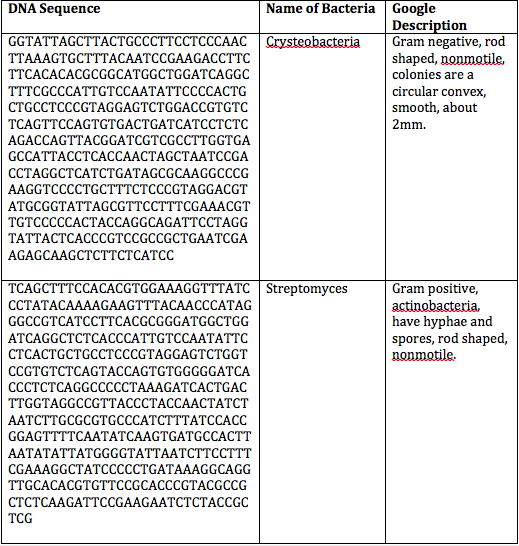
Introduction: The objective of this mini lab was discover which species of bacteria from our transect were put through the PCR reaction and identified based on their DNA sequences.
Procedures: Refer to Lab 2.
Conclusion: Since there were no results for our bacteria after the PCR results were out, we were unable to compare the results with our original observations and assessment. However, the PCR reaction is another method to identify bacteria species other than using a dichotomous key. It is more precise by using DNA sequences. CM
February 28, 2014 Lab 5
Introduction: The objective of this lab was to understand how invertebrates contribute to the earth and observing how simple systems evolved into more complex systems with specialized cells. In this lab, we observed Planaria, nematodes, and coelomates under a dissecting microscope. We also studied invertebrates from our transect that we collected using the Berlese Funnel method.
Procedures: 1. Observe the movement and cross sections of planaria, nematodes, coelomates under a dissecting scope and microscope. 2. Break down Berlese funnel setup and transfer the preservative solution to a petri dish and examine under a dissecting microscope. 3. Identify different groups of invertebrates using a dichotomous key, record in table. 4. Consider what kind of vertebrates may inhabit or pass through your transect.
Results: The movements of the three different invertebrates observed under a dissecting microscope were somewhat similar, but clearly not evolved the same way. The planaria moved in a wave-like motion and this is likely because they do not have a circulatory system, but they do have a digestive tract. The nematodes had a more squirmy movement and were able to move back and forth more quickly than the planaria. This is because they have more muscle and tissue development that allow them to move more. Lastly, the coelomates were much larger and would expand, contract, and completely lift up one end of their bodies. These invertebrates have much more developed muscles and tissues for such large movement.
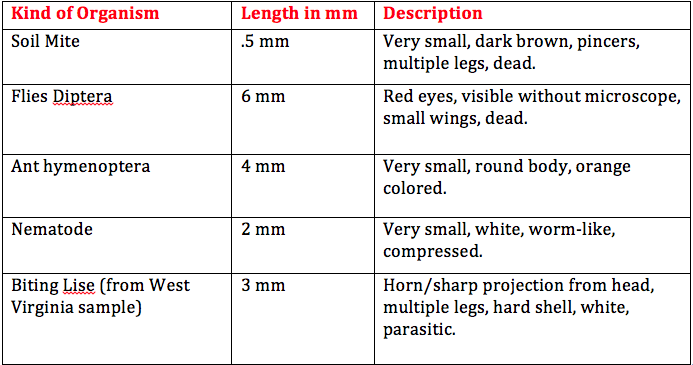
The size range of organisms in our transect was between .5 and 6 mm. The largest organism was the flies diptera and it was visible without using the dissecting microscope. The soil mite was the smallest organism found and they are one of the most common in leaf litter. Other common organisms found in leaf litter include nematodes, ground spiders, millipedes, centipedes, springtails, and more. There are various vertebrates that might inhabit our transect. One species could possibly be the American Robin. It is part of the kingdom Animalia, phylum Chordata, class Aves, order Passeriformes, family Turdidae, genus Turdus, and species T. migratorius. Biotic factors that would benefit the American Robin include trees, berries on the holly bushes, and branches and twigs found on the ground. This species would benefit from abiotic factors such as soil for when they are building their nests. Another vertebrate species that possibly inhabits our transect is the Field Sparrow. It belongs to the kingdom Animalia, phylum Chordata, class Aves, order Passeriformes, family Emberizidae, genus Spizella, and species S. pusilla. Biotic factors that could benefit this species include trees, berries, pine needles, and branches and twigs. Abiotic factors that could be beneficial to them include soil, sunlight, and rain water. Another vertebrate species that could be found in our transect could be the Eastern Gray Squirrel. It belongs to the kingdom Animalia, phylum Chordata, class Mammalia, order Rodentia, family Sciuridae, genus Sciurus, subgenus Scuirus, and species S. carolinensis. Biotic factors that the Eastern Gray Squirrel could benefit from include trees, berries, and leaves. Abiotic factors that could benefit this species include soil and sunlight. Another vertebrate species possibly found in our transect could be the Brown Rat. It belongs to the kingdom Animalia, phylum Chordata, subphylum Vertebrata, class Mammalia, order Rodentia, family Muridae, subfamily Muridae, genus Rattus, species R. norvegicus. This species could benefit from biotic factors such as plant matter on the ground and other organisms found in the soil. Abiotic factors they could benefit from in our transect would be soil and any garbage left by humans. One last vertebrate that could be found in our transect could be the Deer Mouse. It belongs to kingdom Animalia, phylum Chordata, class Mammalia, order Rodentia, family Cricetidae, genus Peromyscus, and species P. maniculatus. Biotic factors that this organism could benefit from include other organisms living in the soil, plant matter on the ground, and berries. Abiotic factors that the Deer Mouse could benefit from include soil and rain water.
Conclusion: The objective of this lab to understand how invertebrates contribute to the ecosystem and to observe how simple systems evolved into more complex systems was accomplished. We studied the invertebrates found in our own transects and identified specific characteristics about each. In the future, the information we discovered in this lab will be applied to draw greater conclusions about our transect and characteristics of this ecosystem.
CM
February 13, 2014 Lab 4
Introduction: The objective of this lab was lab was to identify plants in our transect by observing characteristics of the different organisms and to understand how fungi interact with plants. In this lab we gathered different plant samples from our transect and studied characteristics including vascularization, leaves and specialization, and seeds or reproductive parts. We also observed fungus and learned why they are mutualistic to plants.
Procedures: 1. Obtain about 500g of leaf litter and soil into a plastic bag from the transect. 2. Obtain 5 different plant samples from transect, including pine cones, seeds, flowers, etc. 3. Observe a slide with moss, Mnium, under a microscope and note the height. 4. Observe the cross section of a lily stem under a microscope and find the xylem and phloem. 5. Examine moss leaves and angiosperm leaves. 6. Observe the bryophyte reproductive cycle. 7. Dissect a lily flower sporophyte and find the anther. 8. Dissect the soaking seeds of a flower as well as seeds from the transect. 9. Observe the vascularization, shape, size, and seeds of the plants found in the transect. 10. Examine black bread mold in a petri dish under a dissecting microscope. Observe hyphae, mycelium, sporangia, spores, and rhizoids. 11. Begin setting up the Berlese Funnel by pouring 25 ml of 50:50 ethanol/water into a flask. 12. Tape screening material to bottom of funnel and place funnel into the flask. 13. Put leaf litter sample in funnel and place a 40-watt lamp above and cover with foil.
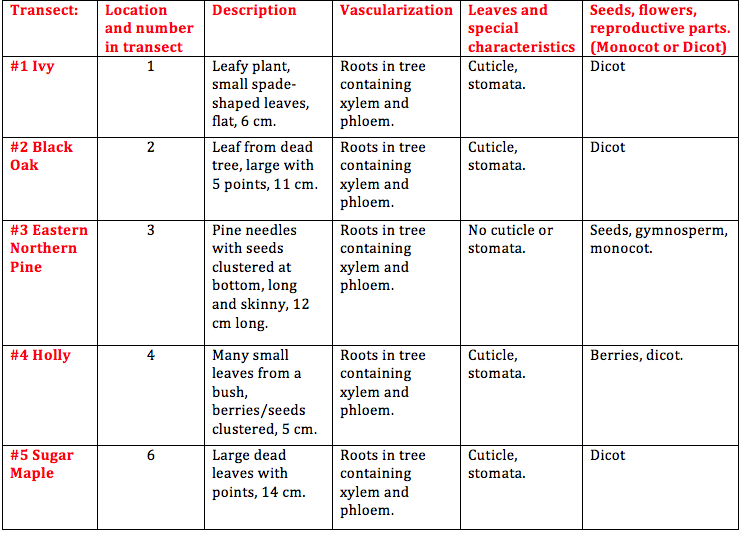
Results: Observations of plants from our transect:
When looking at the fungi under the microscope, we were able to identify fungi sporangia. These are tiny enclosures where spores are formed via meiosis and sometimes mitosis. Sporangia are essential for fungi reproduction because it is where new cells are produced and formed into spores. The fungi I looked at under the microscope belonged to the group zygomycetous.
Conclusion: In our transect, we found leaves from ivy, a black oak tree, a sugar maple tree, holly bush, and needles from an eastern northern pine tree. We were able to observe characteristics of each plant and determine each species. We also observed fungi under a dissecting microscope and examined its characteristics. That being said, the objective of this lab was met. In the future, we will be using our leaf litter sample isolate invertebrates using the Berlese Funnel method and add to our knowledge of known organisms in our transect. CM
February 11, 2014 Lab 3
Introduction: The objective of this lab was to study the bacteria we grew on agar plates and observe characteristics of different bacteria species. We also observed antibiotic resistance involving tetracycline and prepared a PCR reaction that will allow us to use DNA sequences to identify species. Out of the bacteria found on our agar plates, I do not believe any Archaea species would have grown. Archaea grow in extreme environments such as a volcano or the Great Salt Lake and the agar plates are not able to support their lifestyle.
Procedures: 1. Observe hay infusion culture and note any changes in smell or appearance. 2. Count the total number of colonies on each agar plate. 3. Observe differences in plates with vs. without antibiotic. 4. Observe a prepared slide with different kinds of bacteria under a microscope using the 40x and then 100x objective. 5. Make wets mounts from your agar plates- two from the nutrient agar plates and two from the nutrient agar plus tetracycline plates. 6. Use a sterile loop to scrape a tiny amount of bacterial growth from the agar plates onto a slide and add a tiny drop of oil on top of the smear. Observe under the 10x and 40x objective. 7. Make a gram stain by labeling the slides and heat fix the air by passing it through a flame, bacteria side up. 8. Cover the smear with crystal violet over a staining tray for 1 minute, rinse with water from a wash bottle. 9. Cover smear with Gram’s iodine for 1 minute, rinse with water. 10. Decolorize by flooding smear with 95% alcohol for 10 seconds, rinse with water. 11. Cover smear with safranin stain for 20-30 seconds, rinse with water. 12. Blot excess water and let air-dry. 13. Observe sample at 10x and 40x. 14. Choose one nutrient agar plate and one nutrient agar plus tetracycline plate to use for DNA isolation in a PCR reaction. 15. Use a sterile loop to transfer a colony of bacteria to 100 microliters of water in a sterile tube. 16. Incubate for 10 minutes and centrifuge, and then use 5 microliters of the supernatant in the PCR reaction.
Results: Our hay infusion culture looks the same as it did the previous week. There was less water though, but the smell was not as strong and there was no algae or bacteria film on the surface. When observing agar plates, I noticed that the colonies on the plates with nutrient agar plus tetracycline contained larger but fewer colonies and the plates with just the nutrient agar contained many colonies that were smaller. This indicates that on the plates with nutrient agar plus tetracycline, the cells tend to grow on top of each other. Therefore, tetracycline decreases the amount of bacteria, but increases the size. There were no fungi found on our plates. The amount of species of bacteria affected by tetracycline is unknown because there are thousands of bacteria species and they have not all been tested. Tetracycline works by preventing the introduction of new amino acids after binding to a small ribosomal subunit. Bacteria regularly pump tetracycline into their cytoplasm which is how the become infected. Bacteria that are sensitive to tetracycline include chlamydia, mycoplasma, rickettsia, syphilis, leptospirosis, and Lyme disease.
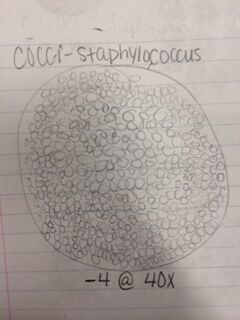
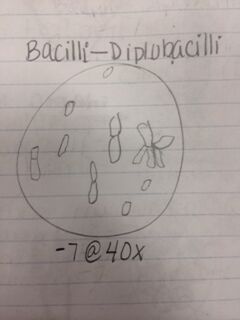
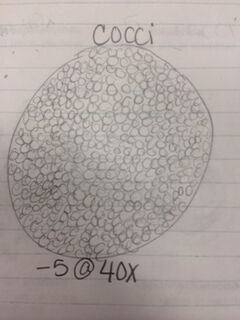
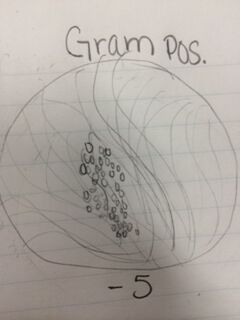
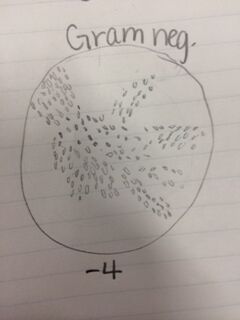
Conclusion: After observing bacteria from our agar plates, we were able to identify two cocci bacteria and one bacilli bacteria as well as determining whether they were gram positive or negative. We also observed the antibiotic resistance to tetracycline and how bacteria grows with its presence. That being said, the objective of this lab was met and I have a better understanding of different characteristics of bacteria, antibiotic resistance, and how to prepare bacterial DNA for a PCR reaction. In the future, we will be able to isolate the DNA from the bacteria in our hay infusion culture to identify DNA sequences. CM
February 8, 2014 Lab 2
Introduction:
The objective for this lab was to identify algae and protists and observe characteristics of these organisms by using a dichotomous key. We used different samples samples from our hay infusion culture and observed them under a microscope to identify them. Based on what we observed, we were able to identify several types of protists with different characteristics by referring to a dichotomous key.
Procedures: 1. Practice using a dichotomous key by making a wet mount from a sample of known organisms and observe under a microscope, study characteristics of different organisms. 2. Observe hay infusion culture and describe appearance. 3. Take samples from different niches within the hay infusion culture for observation. 4. Observe organisms under a microscope and draw pictures of them. 5. Characterize 3 different organisms from 2 different niches. Use the dichotomous key to determine specific characteristics. 6. Measure the size of each organism using the ocular micrometer. 7. Pick one organism and describe how it meets all the needs of life on page 2 in the Freeman text. 8. Prepare 100-fold dilutions of the culture for the agar petri dishes. 9. Label four test tubes of 10 mls sterile broth and obtain four nutrient agar plates and four agar plus tetracycline plates and label those as well. 10. Swirl hay infusion culture and take 100 microliters from it and add it to the 10 mls of broth. 11. Take 100 microliters of broth from tube 2 and inoculate tube 4, swirl to mix. 12. Repeat step 11 two more times. 13. Take 100 microliters from the 10^-2 tube and place of agar plate label 10 ^-3 and spread sample over plate. Repeat on tet plate. 14. Repeat step 13 with the other 3 tubes and plates
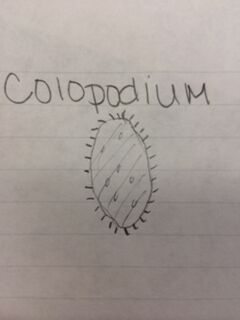
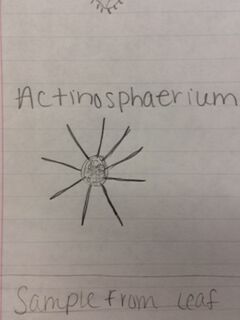
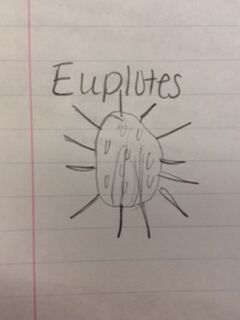
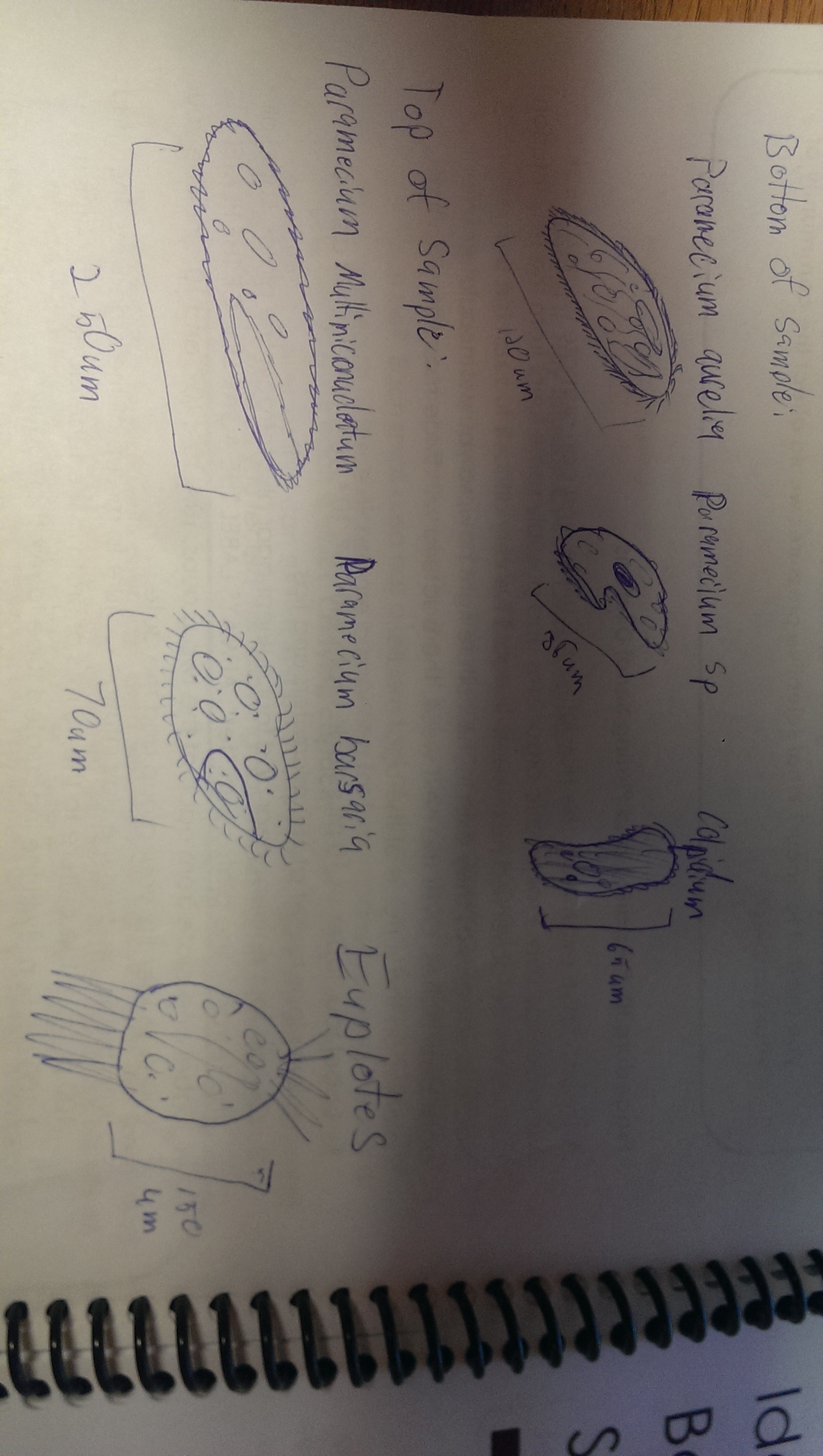
Results: Hay infusion observations - Dark brown water, residue on bottom of jar (sand or dirt), film-like mold or algae on surface of water, smells like dirt, plants and branches mixed in (unsettled). We took one sample from the residue on the bottom of the jar and one sample from a leaf in the middle of the jar. Organisms might differ near versus away from the plant matter depending on the way they eat. Organisms that are heterotrophs intake their food from other organic or inorganic compounds and their niche might be close to a plant where there is more food available. Organisms living in a niche near a plant depend on other organisms for survival. Organisms found on the bottom of the jar include a colopodium, euplotes, and actinosphaerium. The colopodium was 50 micrometers long, colorless, small and oval shaped, and moved around rapidly using cilia. The euplotes was 100 micrometers long, oval shaped, and moved rapidly with lots of long pointy cilia called cirri. The actinosphaerium 70 micrometers long, round shaped with long pointy "spines", colorless, and no movement. Organisms found from a leaf in the middle of the jar include a euplotes and paramecium multimicronucleaum. The euplotes was 100 micrometers long, small and oval shaped, colorless and rapid movement using long pointy cirri. The paramecium multimicronucleaum was 210 mirometers long, long and oval shaped, colorless, and had rapid movement using lots of tiny cilia. Since all of these organisms are colorless, they do not undergo photosynthesis and they are all protozoa. For an example of how one of these organisms meets all the needs of life, the paramecium is a great example. Paramecium obtain energy by using their cilia to sweep in other organisms through and oral groove and into their mouth. Paramecium are unicellular and perform all life functions through the one cell. The paramecium cell contains a genome, which is able to interpret hereditary and genetic information. They replicate asexually and are considered products of evolution because they have evolved from a single common ancestor into a unique species.
Conclusion and Future Plans: In this lab, we were able to identify and observe characteristics of a colopodium, euplotes, actinosphaerium, and paramecium from our hay infusion culture using a dichotomous key. Since our hay infusion was contained in a jar, there were several different niches that organisms had to compete for resources within. This selective pressure affected the composition of our sample and which organisms were able to survive. If the hay infusion culture had been observed for another two months, I would predict that there would be less water and fewer life forms. Resources would eventually become scarce and selective pressure would increase. The objective for this lab was met and we were able to observe and identify different organisms from our hay infusion culture which we were able to use serial dilutions in the future. For the next lab, we prepared agar petri dishes in order to observe bacteria colonies and how they will grow over time. CM
2/6/14, Lab 1 notes
Great job! Make sure you include pics from lab 1 and 2 by Sunday. Also, start working on building a map of your transect to detail your land and where your samples are taken from. We will talk about this more Wednesday. Good work!
AP
January 22, 2014 Successfully entered text into my lab notebook.
CM
January 30, 2014 Lab 1
Introduction:
The objective for this lab was to analyze a 20 by 20 feet transect of land on campus. We studied the ecosystem and identified different life forms as well as biotic and abiotic factors. Based on what we observed, we were able to identify specific life forms including protists, bacteria, plants, invertebrates, and animals in the transect.
Procedures: At transect site: 1. Set up the 20 by 20 feet dimensions of the transect using popsicle sticks 2. Describe characteristics of the transect 3. Determine biotic and abiotic factors 4. Take a sample from the transect using a 50 ml conical tube. Gather soil and vegetation. Hay Infusion Culture: 1. Weigh 10-12 grams of the transect sample 2. Place in a plastic jar with 500 mls of deerpark water. 3. Add .1gm dried milk and mix for 10 seconds 4. Remove top of jar and leave open
Results: The transect my group analyzed was located next the church on Massachusetts Ave. It was forest-like in that it contained pine trees and was completely shaded. The ground was covered by ivy, pine needles, leaves, branches, and pine cones. There were a few different kinds of trees including holly, pine, and maple (no leaves). The trunk of the pine tree was black on one side, possibly having a disease. Biotic factors in the transect include ivy, trees (holly, pine, and maple), pinecones, soil, dead pine needles, small plants, and vines. Abiotic factors include water in the soil, air, and dead matter such as dead pine needles, soil, dead leaves, and pinecones.
Conclusion and Future Plans: In the transect my group evaluated, many different life forms were observed including trees, soil, leaves, and dead matter and other forest qualities. Since our transect has a very diverse amount of life forms, in the future we will be able to identify and observe specific organisms such as types of bacteria, protists, and algae. The objective of this lab was met and we were able to identify characteristics of the transect and take a sample for further evaluation.
CM