User:Brittney Thompson/Notebook/Biology 210 at AU
Zebrafish: Estrogen Mimic
Figure: Data Collected for the Control Fish in the 14 Day Zebrafish Study
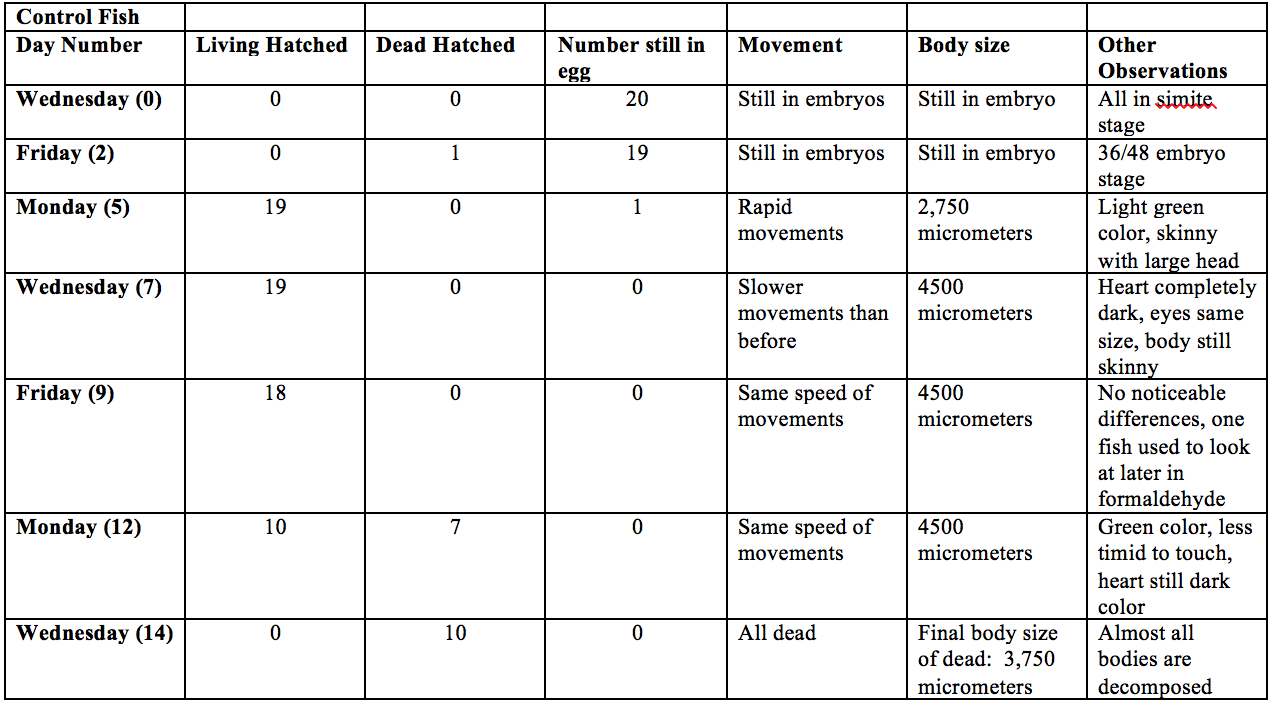
Figure: Data Collected for the Estrogen Fish in the 14 Day Zebrafish Study
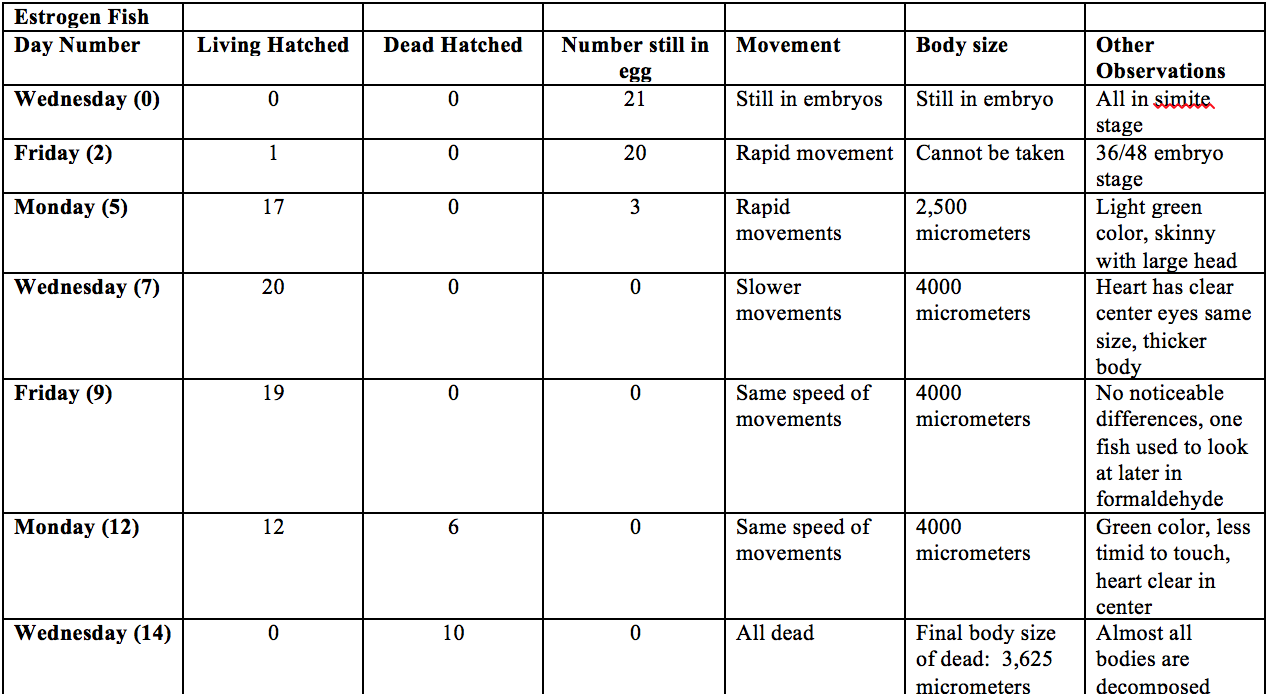
Figure: Data Collected from the Fixed Fish on Day 7

Experimental Method:
40 Zebrafish were split into two groups of 20 and transferred f in separate petri dishes. One dish was filled with 20mL Deerpark water and the other was filled with 20mL 25% estherione. Every other day the fish were observed and their size and death toll was taken. Also the water and estherione was changed to avoid bacteria and food was added after all the fish had hatched. On day seven a fish was taken from each dish and fixed in formaldehyde to help to with accurate sizing.
Week 7: PCR and 16s Sequencing
-During the bacteria study, colonies were taking from two "tet" agar plates and from two non "tet" plates. These samples underwent a PCR reaction and then were implemented into electrophoresis gel to see which contained the 16s gene sequences. From there those colonies in which showed the 16s sequence were sent to get sequenced.
-Once the sequences were returned to us we were able to see the which bacteria each sample belonged to by using the nucleotide BLAST system. This system contains all the known sequences of the world's organisms, and matches your sequence with its closest match.
-From the two samples we received two genetic samples.
1. Sample 1 Sequence: 97% match with Pseudomonas
(NNNNNNNNNNNNNNNNTGCAGTCGAGCGGANGANGGGAGCTTGCTCCTGGATTCAGCGGCGGACGGGTGAGTNNNGNCTA GGAATCTGCCTGGTAGTGGGGGACAACGTTTCGAAAGGAACGCTAATACCGCATACGTCCTACGGGAGAAAGCAGGGGAC CTTCGGGCCTTGCGCTATCAGATGAGCCTAGGTCGGATTAGCTTGTTGGTGAGGTAATGGCTCACCAAGGCGACGATCCG TAACTGGTCTGAGAGGATGATCAGTCACACTGGAACTGAGACACGGTCCAGACTCCTACGGGAGGCAGCAGTGGGGAATA TTGGACAATGGGCGAAAGCCTGATCCAGCCATGCCGCGTGTGTGAAGAAGGTCTTCGGATTGTAAAGCACTTTAAGTTGG GAGGAAGGGTTGTAGATTAATACTCTGCAATTTTGACGTTACCGACAGAATAAGCACCGGCTAACTCTGTGCCAGCAGCC GCGGTAATACAGAGGGTGCAAGCGTTAATCGGAATTACTGGGCGTAAAGCGCGCGTAGGTGGTTTGTTAAGTTGGATGTG AAAGCCCCGGGCTCAACCTGGGAACTGCATCCAAAACTGGCAAGCTAGAGTACGGTAGAGGGTGGTGGAATTTCCTGTGT AGCGGTGAAATGCGTAGATATAGGAAGGAACACCAGTGGCGAAGGCGACCACCTGGACTGATACTGACACTGAGGTGCGA AAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCCGTNNCGATGTCAACTAGCCGTTGGAATCCTTGAGA TTTTAGTGGCGCAGCTAACGCATTAAGTTGACCGCCTGGGGAGTACGGCCGCAAGGTTAAAACTCAAATGAATTGACGGG GGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTCGAAGCAACGCGAANAANCTTANCNNNCTTGACATGCANANAACTT TCCANANATGGATTGGNGCCTTCGGNAACTCTGACNCAGNGCTGCATGNNNGNTCGTCAGCTCGTGTCGTGAGANNNTGN NTNAGTCCCGNTANCNANCGNNNNNCNNGTCCNTANTNNNCAGCNNNTTNTGNNN)
-This species is known to be gram negative, motile, rod shaped, aerobic, and in favor of watery conditions.
2. Sample 2 Sequence: 98% match with Ralstonia
(NNNNNNNNNNTNNNNNNNNNNNAGTCGNNCGGCANCNNGATCTAGCTTGCTAGATTGATGGCGAGTGGCGAACGGGTGAG TAATACATCGGAACGTGCCCTGTAGTGGGGGATAACTAGTCGAAAGATTAGCTAATACCGCATACGACCTGAGGGTGAAA GTGGGGGACCGCAAGGCCTCATGCTATAGGAGCGGCCGATGTCTGATTAGCTAGTTGGTGGGGTAAAGGCCCACCAAGGC GACGATCAGTAGCTGGTCTGAGAGGACGATCAGCCACACTGGGACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAG TGGGGAATTTTGGACAATGGGCGAAAGCCTGATCCAGCAATGCCGCGTGTGTGAAGAAGGCCTTCGGGTTGTAAAGCACT TTTGTCCGGAAAGAAATGGCTCTGGTTAATACCTGGGGTCGATGACGGTACCGGAAGAATAAGGACCGGCTAACTACGTG CCAGCAGCCGCGGTAATACGTAGGGTCCAAGCGTTAATCGGAATTACTGGGCGTAAAGCGTGCGCAGGCGGTTGTGCAAG ACCGATGTGAAATCCCCGAGCTTAACTTGGGAATTGCATTGGTGACTGCACGGCTAGAGTGTGTCAGAGGGGGGTAGAAT TCCACGTGTAGCAGTGAAATGCGTAGAGATGTGGAGGAATACCGATGGCGAAGGCAGCCCCCTGGGATAACACTGACGCT CATGCACGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCCCTAAACGATGTCAACTAGTTGTTGGG GATTCATTTCCTTAGTAACGTANCTAACGCGTGAAGTTGACCGCCTGGGGAGTACGGTCGCAAGATTAAAACTCAAAGGA ATTGACGGGGACCCGCACAAGCGGTGGATGATGTGGATTAATTCGATGCAACGCGAAAAACCTTACCTACCCTTGACATG CCACTAACGAAGCANANATGCATTANGTGCTCNAAAGAGAAAGTGGANNCNNNGCTGCATGGCTGTCGTCAGCNNCGTGT CGTGANNATGTTNGGGTTAAGTCCCCGCAACGAGCNNCANCCCTTGTCTNCTAGTTGCNNN)
-Little is known about the Ralstonia's but they belong to the Proteobacteria genus. Proteobacteria is both non-motile or motile, gram negative, and anaerobic.
Week 6: Identifying Vertebrates
Vertebrates
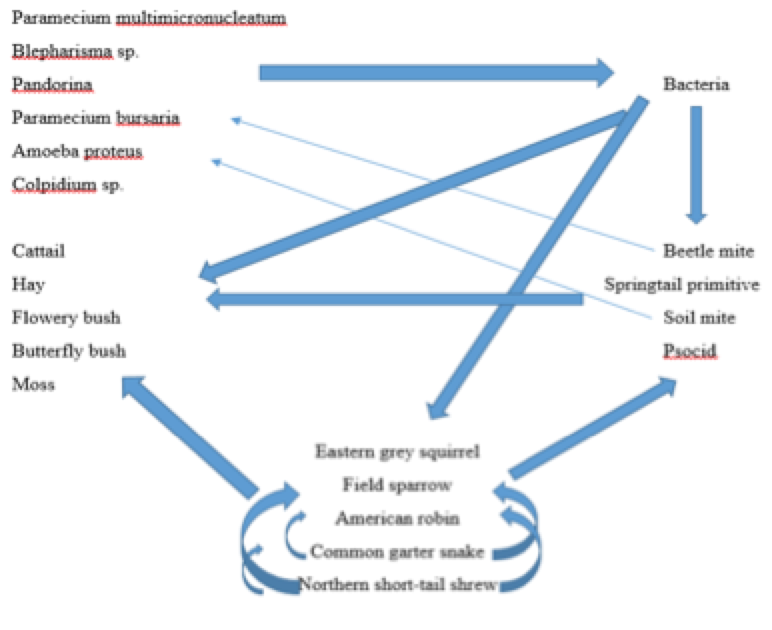
The Common Garter Snake (Thamnophis sirtalis) Phylum: Chordata; Class: Reptilia; Order: Squamata; Family: Colubridae; Genus: Thamnophis; Species: T. sirtalis -Biotic Factors: Tall plants allow for snake to be hidden from predators, also hide them from their prey -Abiotic Factors: Large rocks at edges of marsh allow for the snake to dig a good home, safe and dark.
The Field Sparrow (Spizella pusilla) Phylum: Chordata; Class: Aves; Order: Passeriformes; Family: Emberizidae; Genus: Spizella; Species: S. pusilla -Biotic Factors: Some of the flowered plants have seeds for the sparrow to eat, soil moist so it produces worms/insects for food as well -Abiotic Factors: Water along edges of marsh provide enough supply for sparrow to drink, debris can act as nest builder
The American Robin (Turdus migratorius) Phylum: Chordata; Class: Aves; Order: Passeriformes; Family: Turdidae; Genus: Turdus; Species: T. migratorius -Biotic Factors: Some of the flowered plants have seeds for the sparrow to eat, soil moist so it produces worms/insects for food as well -Abiotic Factors: Water along edges of marsh provides enough supply for robin to drink, debris can act as a nest builder
The Northern Short-tailed Shrew (Blarina brevicauda) Phylum: Chordata; Class: Mammalia; Order: Soricomorpha; Family: Soricidae; Genus: Blarina; Species: B. brevicauda -Biotic Factors: Seeds, worms and insects found on the ground act as nutrition. -Abiotic Factors: Digs into the soft soil from protection and for home, tall grass also protects them from predators
The Eastern Grey Squirrel (Sciurus carolinensis) Phylum: Chordata; Class: Mammalia; Order: Rodentia; Family: Sciuridae; Genus: Sciurus; Species: S. carolinensis -Biotic Factors: Seeds, worms and insects found on the ground act as nutrition. Also moss and fungi found around rocks also can be in the squirrels diet. -Abiotic Factors: Digs into the soft soil from protection and for home, tall grass also protects them from predators
Figure: Food Web of Transect Ecosystem
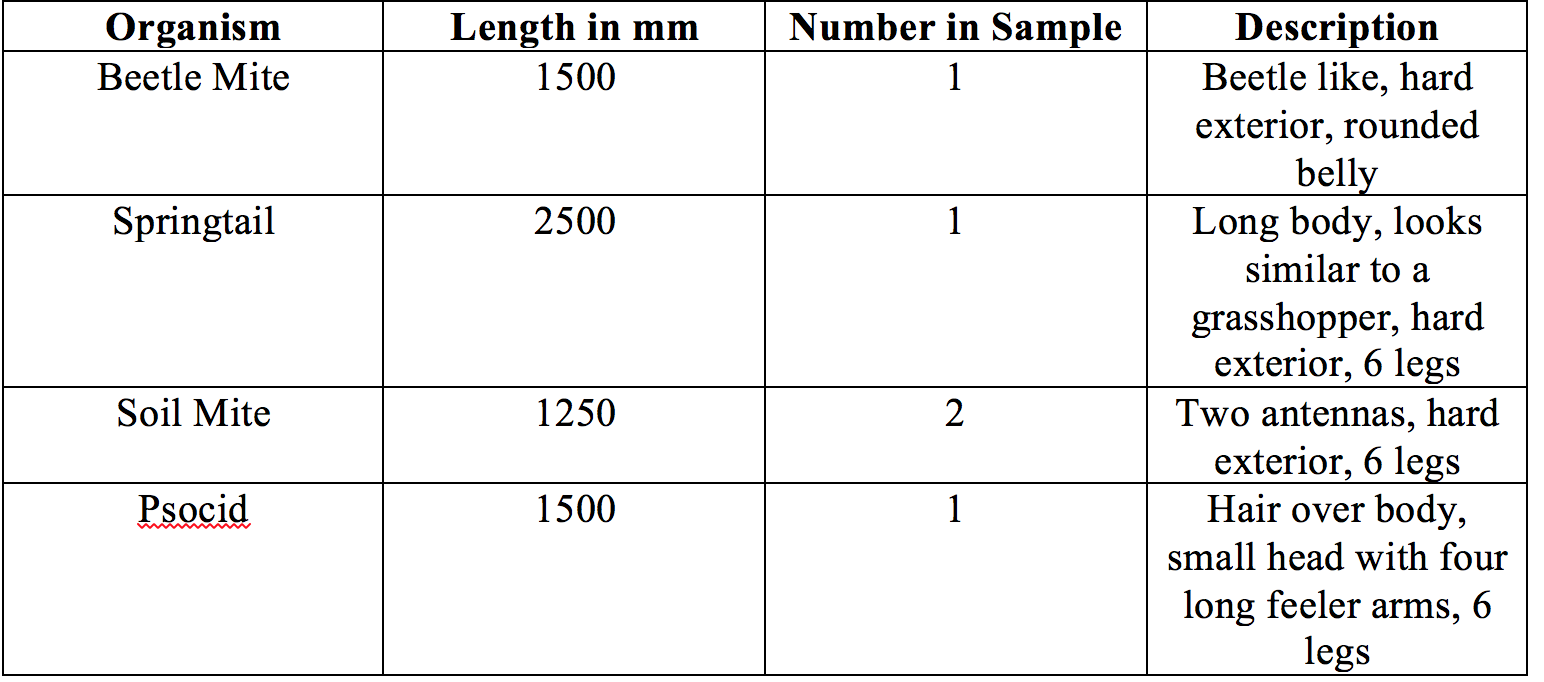
Week 5: Identifying Invertebrates
Materials and Methods: Berlese Funnel: A Ziploc bag was used to collect dead leaves and soil content from the marsh transect. All contents were placed in a funnel, that had a screen so the dirt and leaves could not leave, with an attached conical tube containing 25mL a 50:50 ethanol/water solution. The funnel and conical tube were placed on a ring stand and moved under a 40-Watt lamp. The conical tube was then used to collect the invertebrate samples, all samples were observed under a microscope.
Conclusion: Most of the invertebrates found in the marsh were arthropods, having hard exteriors and segmented bodies.
Week 4: Identifying Plant Life of the Marsh
Five Plant Samples:
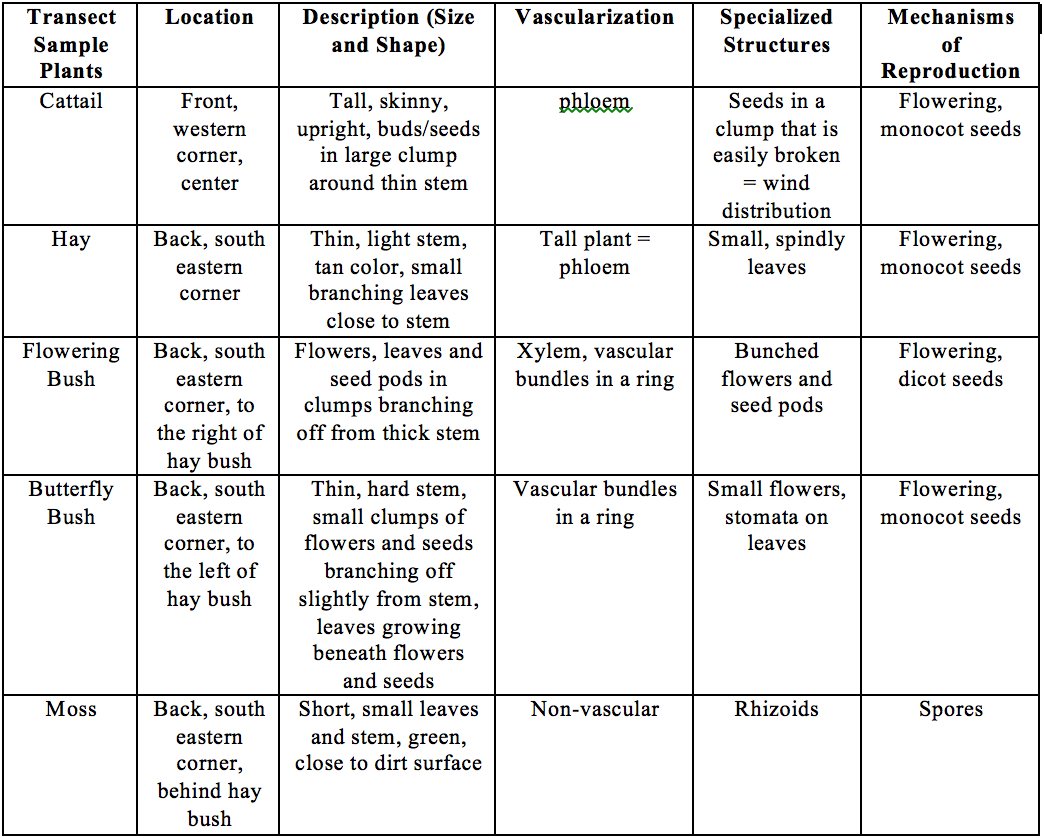
Sample 1: Cat tail, found front left of the transect

Description: A tail, skinny, light, brown plant with a large bud at the end of it. Bud is covered with small seeds with white ends. Vascularization: Contains phloem and xylem Specialized Structure: The bud at the end contains the small seeds light enough to be carried away, this allows for wind distribution of seeds. Reproduction: Flowering plant with monocot
Sample 2: Found towards back of transect

Description: light cream colored, very thin and light weight, small branches with spike like leaves. Vascularization: Contains phloem and xylem Specialized Structure: Thin, spiking leaves branching off, each containing seeds Reproduction: Flowering plant with monocot
Sample 3: Found in the middle of the transect

Description: Tall plant, without leaves until very top were leaves and flowers were mixed together Vascularization: Vascular bundle in a ring, xylem and phloem Specialized Structure: The large bundles of flowers Reproduction: Flowering plant, dicot
Sample 4: Found in the back of the transect

Description: Thin, tall plant. Has small long and slender leaves and clusters of flowers Vascularization: Vascular bundle in a ring, xylem and phloem Specialized Structure: Leaves towards the top of the plants and the small clusters of flowers Reproduction: Flowering plant, monocot
Sample 5: Found in the back corner of the transect, on the ground by the rock.

Description: Green, small leafed, on top of the soil. Barely has height Vascularization: Non-vascular Specialized Structure: has rhizoids, small hairs that branch out like roots Reproduction: Water dependent and uses spores
Plant Life Characteristic Table
Discovering Fungi:
What are the fungi sporangia and why are they important? -Location where spores are held and then released for reproduction.
-We observed a mushrooms, usually part of the basidiomycota branch of fungi. It had small flaps underneath it, they were a darker brown with small dots on it.
Week 3: Identifying Bacteria with Gram Stains and Wet Mounts
Hay Infusion Final Observations: -The smell of the hay infusion has increased since last observed and the water has darkened. All plant life that was originally present has died and there is an increase in the mold.
Materials and Methods: -Serial Dilutions: Four tubes of 10mLs sterile broth were obtained and labeled with 10^-2, 10^-4, 10^-6, 10^-8 along with a micropipette and tips. Then four nutrient agar plates and four nutrient agar plates with tetracycline were gathered labeled- 10^(-3), 10^(-5), 10^(-7), 10^(-9). Those with tetracycline were also labeled with “tet”. The hay infusion was then taken and mixed, 100 microliters was taken from the culture and added to the tube of broth labeled 10^(-2). From the 10^(-2) tube, 100 microliters was removed and placed in the 10^(-4) tube. These steps were repeated for the 10^(-6) and 10^(-8) tubes. From there the serial dilutions were plated on the labeled agar plates. 100 microliters of 10^(-2) broth was pipetted onto the 10^(-3) labeled agar plate and spread carefully with an alcohol-sterilized instrument. Repeat the exact procedure with the 10^(-3) “tet” agar plate. These actions were repeated for the 10^(-4) on the 10^(-5)plates, 10^(-6) on the 10^(-7) plates and the 10^(-8) on the 10^(-9) plates. Plates were placed side up in a rack and incubated at room temperature for a week.
-Gram Stain: Four new slides were collected and labeled again with 10^(-3), 10^(-3) “tet”, 10^(-5), 10^(-5) “tet”. With a sterile loop, a small amount of growth was collected off 10^(-3) and placed on its slide and mixed with a drop of water. The slide was then heated with the bacterial smear facing upwards until water was evaporated. This procedure was repeated for the remaining 3 agar plates. A staining tray was collected and the slides were placed above the tray. Each was covered with crystal violet solution for a minute then rinsed off with distilled water. Then the smears were washed with Gram’s iodine mordant for a minute and again rinsed with distilled water. After the smears were thoroughly washed, they were decolorized with a 95% alcohol wash for 20 seconds. The smears were then covered with safranin stain for 30 seconds and again rinsed with distilled water. Any excess water was removed with kimwipes and the slides were allowed to air dry.
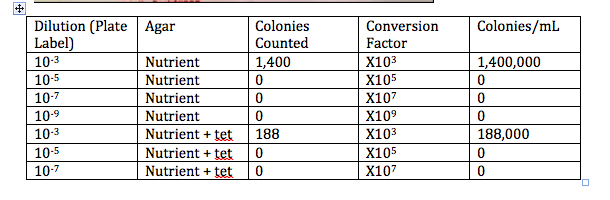
100-Fold Serial Dilutions: Table 1 with Data Collected from Agar Plates
Figure: Agar Plate without "tet"
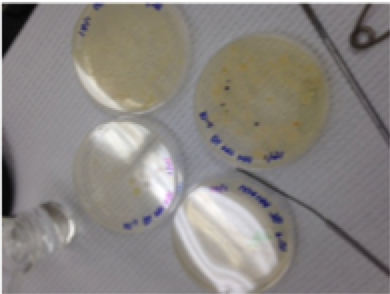
-The plates that contained the nutrient plus the tetracycline contained less bacteria and more fungi than the plates with only nutrient. This indicates that the bacteria grown in my microsystem do not have a resistance to the antibiotic tetracycline. If they would have had a resistance they would have grown more, or about the same on the tet+ plates.
-How tetracycline works is it inhibits the tRNA from attaching the the entrance site (A) of the ribosome which would then synthesize proteins. Tetracycline mostly works against most gram-negative and gram positive bacteria like chlamydiae and parasites. (Chopra, Roberts, 2001).
Bacteria Characterization: Table 2
Picture:  -All viewed in 40x magnification
-All viewed in 40x magnification
Week 2: Hay Infusion Culture
Hay Infusion Culture: During the previous weeks experiment, 11 grams of the transect was placed into a plastic jar with 500 mLs of Deer Park water and .1gm of dried milk. The mixture was slowly mixed with the lid on. After it was placed to the side with its lid removed and not touched until this week. Observations were made when we first returned to the jar after a week had passed.
Observations: The water has become brown and there is a thin layer of mold on top. The sample smells of manure and swampy smell.
Sample 1: Taken from the top layer of the culture
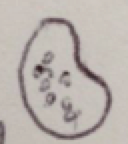
1. 100 um, oblong, floats, pouches within membrane, dark colored
Found to be the Paramecium multimicronucleaum
2. 450 um, pink colored, long stripes present within membrane, thin stick like.
3. 70 um, green colonies, 7 large colonies surrounded by several smaller ones, non-moving
Sample 2: Taken from the bottom layer of the culture
1. 50-60 um, dark colored, moves fast, vacuoles visible within membrane.
Found to be the Paramecium bursaria

2. 20-30 um, changes shape, floats, colorless
Found to be the Amoeba proteus
3. 60 um, fast, oval shape, colorless, one large vacuole visible
If the Hay culture were to grow for another two months I believe there would be a growth in some of the bacteria currently present. However, the number will stop after the ecosystem reaches its carrying capacity for that specific niche of the marsh. I chose bacteria to grow best in this environment because of their small size and relative ability to live in countless places, they will have the most fitness in the culture. Other creatures are bound to run out of resources before the bacteria, and soon the bacteria will hold majority due to the selective pressures.
Week 1: Examining the Marsh for the First time
Location: American University, to the right of the Kogod Business school near Massachusetts Ave.
Description: The marsh at AU was a 20 by 20 plot of land that consisted majorly of tall plants, several rocks and damp soil. The transect is situated between two sidewalks which students use every day to get to their classes. Our transect has heavy foot traffic, just during observation 10+ students passed by. The water drain is situated right at the edge of the plot making our transect very moist from the water running into the drain. Most plant life appeared to be dead, all having a light brown color.
Figure: Bird's Eye View of Marsh from Google Maps
Abiotic Factors: -Rocks -Dirt -Light -Water -Sewage drain off to the left
Biotic Factors: -Cardinal Flower (upper right) -Grass -Moss -Clovers -Butterfly bus (upper right) -Cat tails (lower center)
Works Cited: Chopra I, Roberts M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiology and Molecular Biology Reviews 2001;65(2):232-260. doi:10.1128/MMBR.65.2.232-260.2001.