User:Brittney Nalty/Notebook/Biology 210 at AU
March 18, 2014-LAB 6-Performed February 27, 2014
Goal/Objective: Learn the stages of embryonic development; compare embryonic development in different organisms; set up an experiment to study the effect of alcohol on embryonic development in zebrafish.
Methods: Procedure 1 Compared embryology features and ecological aspects of starfish, frog, and chick development based on tables and pictures that were provided. Procedure 2 (Zeberafish experiement)- 1) Observed and determined the developmental stage of zebrafish embryos that would be used in the experiement. 2) Three groups of 20 embryos were separated, one control which contained 20mls of Deerpark water, one group with 1.0% EtOH and one group with 2.0% EtOH. 3) The embryos/larvae were observed for a week, to see the effect that alcohol had on their development.
Data/Observations:File:Data for zebrafish.pdf -Table 1: data for life of zebrafish; File:Qualitative data for zebrafish.pdf-Table 2: Qualitative Data for zebrafish
Conclusions: In the end, it was interesting to note that the control group died off before the treatment groups, this could have not been due to experimental issues, but perhaps due to the amount of sunlight that was received by the control group. Just as predicted, the treatment group developed differently than the control group, and in the end, the group in the 2% EtOH had smaller heads, eyes, and body sizes than the other two groups. This could be an indication of the effect that the difference of concentration of alcohol has on the zebrafish.
March 7, 2014-Identification of bacteria from transect
Goal/Objective: Identify the bacteria that were found in the transect.
Methods: The DNA was sequenced from the 16S rRNA gene with the PCR reaction performed in lab 3. Using the results, the sequence was entered into the NCBI blast and the different bacteria were identified. The sequences were as follows:
Sequence 1: TGNTCAGTTTGAACGNTGGCGGCATGCCTTACACATGCAAGTCGAACGGCAGCACGGAGCTTGCTCTGGTGGCGAGTGGCGAACGGGTGAGTAATATATCGGAACGTACCCTGGAGTGGGGGATAACGTAGCGAAAGYTACGMTAATACCGCATACGATCTAAGGATGAAAGTGGGGGATCGCAAGACCTCATGCTYSTGGAGCGGCCGATATYTGATTAGCTAGTTGGTAGGGTAAAAGCCTACCMAGGCATCGATCAGTAGCTGGTNTGAGAGGACGACCAGCCACACTGGAACTGAGACACGGTCCAGACTCCTACGGGAGGCAGCAGTGGRGAATTTTGGACAATGGGCGAAAGCCTGATCCAGCAATGCCGCSYGAGTGAAGAAGGCCTTNNGGTYGTAAAGCTCTTTTGTCANGGAAGAAACGGTGNARRGCTAATTTNCTTTGCTAATGACGGWACCWGAAGAATAANCANNNGCTAANN
Sequence 2: AGCGGTAGAGATTCTTCNGAWTCTKRAKAGCGGMGTRCRGRTKMRGAACACGKRWGCAASCTGSCTTTRTSGSGGGGATARCCTTTCKAAAGGAAGATTAATMCCCCATAATATATTAARNNNNATCASKKGAYMTTNNNGMMAASTCCGGTGGRWAAWGATGGGCWCGSRCAAGATTAGWKAGWTGGTAAKGTRRCGGCWANCCAAGTYMGTGATCTTTATGGGGCMTGAKAGGGTGATCCCCCACWCTGGTAMYGAGACMCKGACCAGACTCSTACRGKASGCAGCAGTGAGGAATATTGGACWATGGGTGARAGCCTGATCCMGCCATCCCGCGTGAASGATGACGGCCCTATGRGTTGTAATCTTCTTNTGTATWTGGATAAACCTTNNCNACKGT
Sequence 3: TCNAAACAGCAAAGTATTAATTTACTGCCCTTCCTCCCAACTTAAAGTGCTTTACAATCCGAAGACCTTCTTCACACACGCGGCATGGCTGGATCAGGCTTTCGCCCATTGTCCAATATTCCCCACTGCTGCCTCCCGTAGGAGTCTGGACCGTGTCTCAGTTCCAGTGTGACTGATCATCCTCTCAGACCAGTTACGGATCGTCGCCTTGGTGAGCCATTACCTCACCAACTAGCTAATCCGACCTAGGCTCATCTGATAGCGCAAGGCCCGAAGGTCCCCTGCTTTCTCCCGTAGGACGTATGCGGTATTAGCGTTCCTTTCGAAACGTTGTCCCCCACTACCAGGCAGATTCCTAGGCATTACTCACCCGTCCGCCGCTGAATCAAGGAGCAAGCTCCTCTCATCC
Data/Observations: Two of the bacterium were able to be identified. The chart that was made in lab 3 has been updated with the identification of the bacteria. File:Updated table.pdf
Conclusions: It was interesting to notice that bacteria on the 10^-3 nutrient was unidentifiable, this could be something that could potentially be looked at in future studies to attempt to identify the bacteria. Also some findings were inconsistent, as the pseudomona is a gram negative bacterium and it was observed to be gram positive. This is something that could be re-analyzed for confirmation.
February 23, 2014-LAB 5-Performed February 20, 2014
Goal/Objective: Analyze the invertebrates collected with the Berlese Funnel
Methods: Transferred the preservative solution to a Petri dish (one from the top and one from the bottom of Berlese Funnel) and examined under a dissecting microscope.
Data/Observations: The size range that was found of the organisms that were measured was really small. The organisms from both the top and bottom of the Berlese funnel were microscopic, and appeared as a "speck" or nothing at all with the naked eye. All of the organisms required magnification to see, and they were all organisms that were found in the soil. The following table provides information about the three organisms File:Invertebrates.pdf
Figure 2: Springtail Primitive 
Conclusion: In conclusion, it was realy interesting to see the kinds of invertebrates that live in the tall trees transect and to observe how microscopic they are. BN
February 13, 2014-LAB 4-Performed February 6, 2014
Goal/Objective: The goal of this lab was to understand the characteristics and diversity of Plants and to appreciate the function and importance of Fungi.
Methods: Procedure 1 (Collecting 5 plant samples from the transect): 1) one bag was filled with leaf litter from the transect (including soft soil and dead leaves) which would be used to set up the Berlese funnel 2) Representative samples from five plants in the transect were taken and placed in a bag. 3) the five plants were described (table 1 below) Procedure II (Plant Vascularization): 1) Observed the moss, Mnium 2) examined the cross section slide of the lily stem 3) vascularization of each plant from transect was described (table 1) Procedure III (Plant Specialization): 1) Leaves of the moss and angiosperm were observed. 2) Shape, size, cluster arrangement of leaves from transect plants were described (table 1) Procedure IV (Plant Reproduction): 1) Observed the moss, "Polytrichum" 2) Identified whether the seeds from the plants of the transect were either monocot or dicot (table 1) Procedure V (Observing Fungi): 1) examined black bread mold under a dissecting microscope Procedure VI (Setting up the Berlese Funnel to Collect Invertebrates): 1) about 25ml of the 50:50 ethanol/water solution was poured in a flask, 2) a piece of screening material was taped into the bottom of the funnel 3) the funnel was placed into the neck of the square-sided bottle 4) the leaf litter sample was placed in the top of the funnel 5) a 40watt lamp was placed above the funnel with the bulb about 1-2 inches from the top of the leaf litter and everything was covered with foil
Data/Observations: Plant vascularization, specialization, and reproduction of plants 1-5 from our transect are all described in the following table File:Table 1-transect plants.pdf. Figure 1: Plants samples 1-5 [[1]]
When observing the moss, it measured to be about 2" in height, while the lily was a lot taller, measuring to be about 2' tall. This was strong evidence of the effects of having vascularization. With the stems of the lily containing the pith, protoxylem, xylem, phloem, sclerenchyma, cortex, and epidermis, nutrients and water are better able to be transported throughout the plants causing these huge variation in plant height when comparing the moss to the lily.
According to [[2]] and [[3]] Fungi sporangia are involved in asexual reproduction of spores. The asexual sporangia are important for the reproduction of fungi. Below in figure 2, an image of fungi that was observed in lab. It is evident that is fungi because of the visibility of the sporangia cells (spores). These cells are on the tip of the mycelium or hyphae. Figure 2: Fungi [[4]]
Our Berlese funnel contained mostly dead leaves and soil from our transect. There was no visible evidence of living organisms. Figure 3: Berlese funnel [[5]]
Conclusions: The plants that were found in the transect were not abled to be appropriately named, other than the American Holly. It was a challenge to fully categorize the plant samples, as most of the leaves were dead. It would have been interesting to get more of an understanding of the angiosperm that contained the dead flower bud, perhaps in the spring. Witnessing the effects of vascularization was interesting, it was really brought into perspective when observing the differences in the moss and lily plant. I am also very curious to observe the invertebrates form the leaf litter of the transect since there were no life forms seen with the naked eye. BN
February 10, 2014-LAB 3-Performed January 30, 2014
Goal/Objective: Understanding characteristics of bacteria, observe antibiotic resistance, and understanding how DNA sequences are used to identify species. It was predicted that there would be no evidence of growth of Archaea species on the agar plates because of there extremophile properties. When observing the microorganisms in the hay infusion culture, it was predicted that the appearance and smell may be different from the following week, because the nutrients could have been eaten by any living organisms. Furthermore, these living organisms could no longer be living because of the lack of nutrients, causing the smell to change.
Methods: Procedure I (Quantifying and Observing Microorganisms): 1) Counted the total number of bacteria colonies on each agar plate. Procedure II (Antibiotic Resistance): 1) Looking at results form table 1, made from procedure II, and the following questions were answered: Do you see any differences in the colony types between the plates with vs without antibiotic?; What does this indicate?; What is the effect of tetracycline on the total number of bacteria? Fungi?; How many species of bacteria are unaffected by tetracycline? 2) Find out how tetracycline works and the types of bacteria that are sensitive to this antibiotic. Procedure III (Bacteria Cell Morphology Observations): Observing prepared slides of bacteria- 1) obtained a prepared slide containing different types and shapes of bacteria. 2) Observed stained area of the slide with lower objective of microscope. 3) Centered on the areas and observed with the 40x objective. 4) proceeded to use the 100x oil immersion objective lens using a small amount of oil.- Observed three samples of microorganisms- 1) Chose two samples from the nutrient agar plus tetracycline plate, and one from the nutrient agar plate (the three chosen were the most densely populated, there was not a fourth to observe). 2) Used a compound microscope to observe the cells 3) Made a wet mount 4) Prepared a gram stain of each sample. -Preparing a wet mount: 1) A tiny amount of growth from the surface of the agar plate was scraped. 2) the sample was mixed into a drop of water on a slide. 3) A cover slipped was placed over the drop 4) The wet mount was observed under the 10x and then switched to the 40x objective.- Preparing a gram stain: 1) The slides were labeled. 2) A drop of water was added to a slide, and a small amount of the colony was smeared in the drop. 3) Slide was dried by passing it through a falme three times with the bacteria smear side up. 4) The smear was covered with crystal violet for one minute (over a straining tray) then rinsed with water. 5) The smear was covered with Gram's iodine mordant for one minute, then rinsed gently with water. 6) The smear was flooded with 95% alcohol for 10-20 seconds to decolorize the slide, then rinsed gently. 7) excess water was blotted with a paper towel. 8) the gram stain was observed underneath the microscope. Procedure IV (PCR preparation for DNA sequence Identification: 1) To amplify the 16S rRNA gene, a single colony of bacteria was transfered to 100 microliters of water in a sterile tube 2) It was incubated at 100°C for 10 minutes and put in the centrifuge. 3) 5 microliteres of the supernatant was used in the PCR reaction.
Data/Observations: Table 1: 100-fold Serial Dilutions Results File:Dilution.pdf
Figure 1: Agar plates [[6]]
As seen in the image above, the plates that contained only nutrients produced more of a lawn growth of bacteria, whereas the the ones that also had tetracycline, produced visible colonies. Only the 10^-3 and 10^-5 plates of both with and without tetracycline had visible bacteria growth. With the plates that had the antibiotic, there was clearly less growth, as we were able to count the number of colonies present. This indicated that the colonies that did grow, were the ones that were immune to the tetracycline. Because the plates that grew lawns did not have tetracycline, the colonies grew in large quantities and grew together. Tetracycline prohibits enzyme reactions that are important for bacterial cells, essentially stopping protein synthesis. Tetracycline binds to the 30S ribosome of the bacteria and prevents the tRNA from binding to the RNA. Tetracycline has been used to treat a variety of bacterial infections, however, over the years, immunity to this antibiotic has developed, and it is no longer used to treat staphylococcal, streptococcal, or pneumococcal infections. The bacteria that are sensitive to this antibiotic include are Escherichia coli, Haemophilus influenza, Mycobacterium tuberculosis, and Pseudomonas aeruginosa [[7]]
Table 2: Bacterial cells observations File:Colony observations.pdf
The following images are of the cells underneath the dissecting microscope (figure 2), and the gram staining images (figure 3 and 4)
Figure 2: Colony from tet plate 10^-3 under dissecting microscope [[8]]
Figure 3: Gram stained bacteria [[9]]
Figure 4: Gram negative bacteria [[10]]
Conclusions: Overall, it was interesting to really observe the different characteristics of bacteria and to see the effects that antibiotics have on cell development and growth. It will be interesting to see what comes of the PCR reactions and to be able to identify the species of bacteria that are living in our transect. BN
January 29, 2014-LAB 2- Performed January 23, 2014
Goal/Objective: During lab 2, the hay infusion culture was observed and serial dilutions of the hay infusion were prepared and plated.
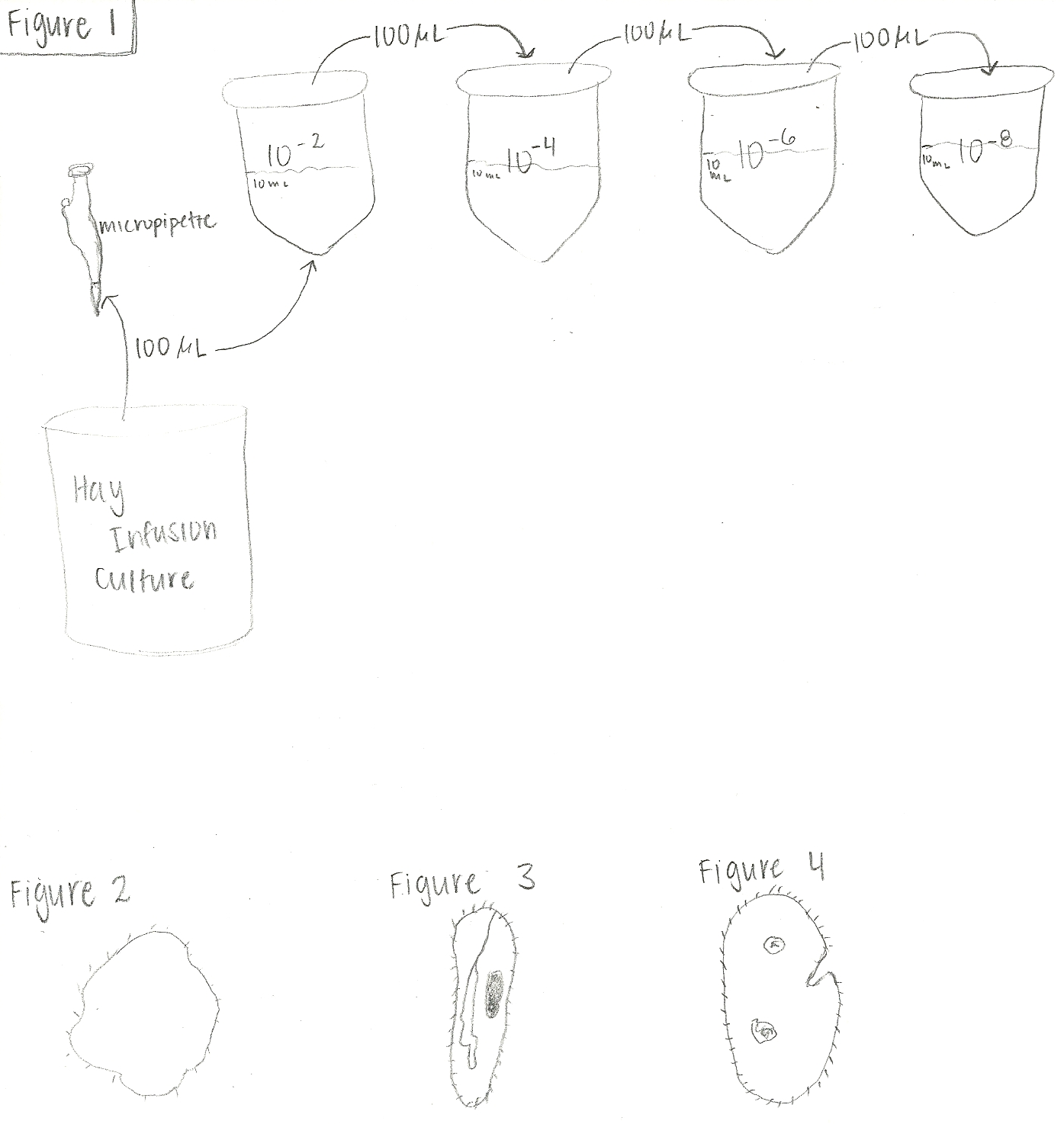
Methods: Procedure I (examining the hay infusion culture): 1) Without disturbing the culture, the plastic jar was brought over to our group's work station, and examined using the naked eye and nose. 2) A few samples were taken from the culture, and observations of organisms from two different niches were made under the microscope. 3) Organisms were identified using the Dichotomous Key. Procedure II (preparing and plating serial dilutions): 1) four 10mls sterile broth tubes were obtained and labeld 10^-2, 10^-4, 10^-6, and 10^-8. 2) The hay infusion culture was swirled to mix all the organisms up and using a micropipeter, 100 microliters were taken from the culture and added to the tube labeled 10^-2 (1:100 dilution). This tube was then swirled well. 3) 100 microliters of broth were taken from the 10^-2 tube and placed in the 10^-4 tube (1:10,000 dilution). This tube was then swirled well. 4) This was repeated two more times to make the 10^-6 and 10^-8 dilutions (figure 1 below). 5) Four nutrient agar and four agar plus tetracycline plates were obtained and properly labeled. The agar plates were labeled with "10^-3, 10^-5, 10^-7, and 10^-9". 6) 100 microliters was taken from the 10^-2 tube and placed on the surface of the 10^-3 nutrient agar plate, and the sample was carefully spread on the plate. 7) This was repeated on the tet plate labeled 10^-3. 8) steps 6 and 7 were repeated with content from 10^-4 tube being spread on the 10^-5 plates, 10^-6 on 10^7 plates, and 10^-8 on 10^-9 plates. (NOTE: SORRY, I TRIED SO MANY TIMES TO GET THIS IMAGE SMALLER, nothing worked)
Data/Observations: Examining the hay infusion culture: Of the undisturbed hay infusion, it smelled of "outside after the rain". The leaf that we had placed in the jar was closer to the bottom where there was dirt/sand layer. On the top of the culture, was a dirt film layer that seemed to be floating on the top. There were not any apparent life that I could see with my naked eye. Microscopic observations: Two samples were taken from the hay infusion culture, one from the top and one for the bottom layer. There was not much life from the top where the film of dirt was. An unclassifiable motile organism was found (figure 2 above). It was spherical in shape and was about 120-140micrometers in diameter. From the bottom layer, there were more observed life form. Paramecium caudatum (200micrometers long) were witnessed (figure 3 above). Also there were a few Colpidium (figure 4 above) moving around that were about 100 micrometers long. The Colpidium moved rather quickly and appeared to have oral grooves. There were green plant-like substances in the same field of view that the Colpidium "grabbed" on to and drug it through the field of view for a while and then dropped it off. This was more than likely how the Colpidium obtained its energy to stay alive and reproduce, by feeding on the green substance. This organism was unicellular and appeared to have small organelles within its unicellular entity. There were several Colpidium moving throughout the field of view, so it was quite evident that some replication had taken place.
Conclusions: It was interesting to witness that more life was present in the bottom layer than in the top layer of the culture. This was more than likely due to the leaf that was near the bottom, or the dirt/sand that was in the bottom of the jar. The leaf and other things in the dirt/sand provided the living organisms with more nutrients which allowed them to live and reproduce. If the hay infusion culture had been observed for another two months, I would predict that there would be even more living organisms in the bottom layer because of the reproduction that would take place. However, once all of the nutrients were to run out, the organisms would die off, as they probably did in the top layer of the culture. BN
January 25, 2014
I visited my transect on Thursday (Jan 23rd) afternoon. The short bushes were covered in snow. The bird's nest in one of the taller trees had snow on top of it. I did not see any life form at the location of the transect, but a squirrel was near by. BN

January 17, 2014-LAB 1-Performed January 16, 2014
Group 3-TALL TREES
Goal/Objective: The goal of the lab was to visit the transect and describe the general characteristics of the transect (location and topography, etc.). Additionally, it was necessary to list abiotic and biotic components of the transect. Finally, collect a sample from the transect to create a hay infusion culture.
Hypothesis: Upon discovery that my transect had been labeled as "tall trees", I hypothesized that the 20 by 20 foot transect would consist of several tall trees.
Data/Observations: I was introduced to my transect, which was located near Bender arena. It consisted of many short bushes with dead leaves and tall bare trees. It is surrounded by evergreen, more specifically, Holly trees were present. It was interesting to note that the transect was half shaded due to the tall buildings. 
Biotic components- short plants, tall plants, weeds, squirrel, bush, bacteria Abiotic components- two lamp posts, sprinkler head, concrete pathway, trash, cigarette
Methods (making hay infusion culture): 1) 10-12 grams of the transect sample were placed in a well-labeled plastic jar with 500mls of deerpark water. 2) 0.1grams of dried milk was added to the jar and gently mixed for 10 seconds. 3) the top of the jar was removed and the open jar was put in a safe place in the lab.
Conclusion: Since the life of the trees and bushes in our transect were in poor to no condition, if I were to collect a sample from my transect again, I would collect one just as we did, from right in the middle of the transect, but I would also collect another along the edge near the holly trees. This would allow us to see if there would be a difference in the organisms between the two niches. I would be curious to observe how the sunlight varies in that area during different times of the day. I think that because our transect appears to be blocked from an ample amount of sun by the tall buildings, this has a great effect on the amount of photosynthesis that is happening in this area. BN