User:Brigette D. Black/Notebook/Brigettes Notebook/2009/07/26/Kinesin Activity with Nanodrop
Today I decided to try a kinestics assay! Slightly different though this time around. Koch had mentioned previously that I should try the assay with about 1 microtubule to 1 molecule of kinesin. The trouble is, we don't know precisely how many tubulin are in a microtubule, so here is some math to give a rough idea:
- Microtubule = 25 nm diameter x 60 um in length
- 1 tubulin dimer = 3nm x 8nm
- Surface area Microtubule = pi*diameter*length = 3*25*60*10^3 nm = 4.5*10^6 nm^2
- Surface area tubulin = 24 nm^2
- number of tubulin = SA MT/SAtubulin = 187,000 tubulin/MT
So by my crappy estimation, I just called it about 10^5 tubulin/MT.
Now, kinesin and tubulin have about the same molecular weight (about 55kDa) so I preformed the experiment with a concentration of 10^5 more tubulin than kinesin (or about 1 Mt to 1 kinesin)
- Tubulin: 5uL at 5 mg/mL (90uM)
- =>+ 0.5uL BME + 9.5 uL BRB80T (30uM tubulin)
- =>+14uL ATP (30uM tubulin, 50mM ATP)
- Kinesin: .25uL at 1mg/ml (18uM)
- => + 488uL BRB80T (.009 uM = 9nM)
Add 2uL tubulin+ATP solution to 38 uL Kinesin
Final Concentrations: (in 40uL)
- Tubulin = 1.5 uM
- Kinesin = 8.5 nM
- ATP = 2.5mM
So with these measurements I have about 100 kinesin per microtubule, not quite the one to one ratio, but not completely useless to look at.
I mixed 24 uL of the MT+ATP solution with 456 uL of the kinesin. When a time interval would com, I would take 40 uL of this sample and quench the reaction in a cuvette with 200 uL of perchloric acid. I then added 200 uL of the malachite green dye, waited 10 minutes for it to develop, then took a measurement at 640, 645, 650, 655, and 660 nM. I blanked on an empty cuvette, so background from the MTs, ATP, or kinesin are subtracted in the following measurements.
I made the following cuvettes:
- Cuvette 1: MT+ATP +38 uL BRB80
- Cuvette 2: MT+ATP +K ( 0 mins)
- Cuvette 3: MT+ATP +K ( 10 mins)
- Cuvette 4: MT+ATP +K ( 20 mins)
- Cuvette 5: MT+ATP +K ( 30 mins)
- Cuvette 6: MT+ATP +K ( 45 mins)
- Cuvette 7: MT+ATP +K ( 60 mins)
- Cuvette 8: MT+ATP +K ( 90 mins)
- Cuvette 9: MT+ATP +K ( 120 mins)
- Cuvette 10: MT+ATP +38uL BRB80 ( 120 mins)
- Cuvette 11: MT+ATP +K ( 24 hrs)
- Cuvette 12: MT+ATP + 38uL BRB80 ( 24 hrs)
- Cuvette 13: MT+ATP + 38uL BRB80 ( 48 hrs)
- Cuvette 14: MT+ATP +K ( 48 hrs)
- Cuvette Time Absorbance at 640, 645, 650, 655, 660 nm
- 1 0 0.116, 0.110, 0.101, 0.089, 0.081, 0.08
- 2 0 0.119, 0.113,0 .105, 0.092, 0.083, 0.081
- 3 10 0.106, 0.098, 0.0910, .079, 0.074, 0.070
- 4 20 0.107, 0.013, 0.097, 0.079, 0.075, 0.069
- 5 30 0.117, 0.105, 0.098, 0.084, 0.082, 0.079
- 6 45 0.116, 0.104, 0.098, 0.084, 0.079, 0.078
- 7 60 0.117, 0.106, 0.099, 0.086, 0.081, 0.078
- Redo Calibration (MT+ATP 1hr) +K (0min): 0.111, 0.098, 0.090, 0.075, 0.071
- 8 90 0.129, 0.117, 0.113, 0.098, 0.094, 0.089
- 9 120 0.134, 0.121, 0.112, 0.098, 0.098, 0.091
- 10 120 0.121, 0.110, 0.102, 0.086, 0.083, 0.080
- 11 24hr 0.133, 0.125, 0.166, 0.103, 0.10, 0.089
- 12 24hr 0.107, 0.098, 0.089, 0.077, 0.074, 0.065
The 48 hour samples did not make it, I miscalculated the total volume I would need and was left with only 30 uL of the kinesin+MT mixture, so I didn't measure it since the results would not be similar.
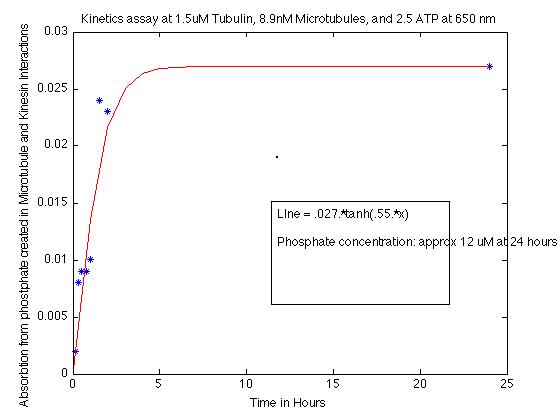
In general, this assay seemed to have a very very low reaction, really really low. When the redo calibration is subtracted from the later measurements, the absorbances are about 0.008, which relates to about 1-5 uM Pi. At 24 hours, the absorbance is about 0.027, which relates to a Pi concentration of about 12 uM. So it is still pretty low. But at lest it is active!
Also, I have noticed that malachite green is very quick, and changes very quickly even at 10 minutes. By measuring even 30 seconds too late, absorbances seem to be about 0.01 larger. So it is very possible that much of the data was taken slightly later than intended and thus artificially high, or slightly earlier and artificially low. So in the future I need to be hyper-vigilant about getting them in on time, or to measure a sample and see where the malachite green begins to decelerate and shot for that developing time.
When I did this assay previously, I saw results directly from kinesin activity. I used about 5x more tubulin than kinesin (or about 20,000 kinesin / microtubule). This may be a little more kinesin than necessary, but it would be good to find a happy balance between more kinesin and microtubules.
My idea is to do this assay again, but this time with maybe 1 tubulin to kinesin (about 100,000 kinesin / MT). Hopefully this will give some nice results!