User:Andy Maloney/Notebook/Lab Notebook of Andy Maloney/2010/07/28/Caseins
Beta casein
First attempt
So I prepared a slide as usual with beta casein passivation. However, it has been 13 minutes since I removed the slide from the hot plate to the microscope and I still am not able to see motility. This could mean that my kinesin is bad but, I used this same kinesin yesterday to look at whole casein passivation. I'm not sure what is going on. I'll keep it on the microscope for a little longer to see if motility occurs. If it doesn't, then I'll have to try a different vial of kinesin.
After 30 minutes, still nothing. Now, I cannot remember when I first did the beta casein solution, if I filtered it through a 0.2um filter or not. And of course, I didn't write the damn thing down in my notebook. I have no clue as to if this is the reason why my assay is not working or not but it may be. I'm going to try a different vial of kinesin first as this is the easiest thing to do. If is still doesn't work, then I'll try making another solution of 0.5mg/mL beta casein in PEM but I won't filter it.
Second attempt
The second attempt I tried a new aliquot of kinesin. This did seem to work however, with a caveat. I'm only seeing motility on the slide and not on the slip. Trying to track microtubules on the slide is going to be impossible due to the massive amount of noise caused by the other microtubules in solution.
I did take a look at my first attempt again to see if there was any motility going on on the slide. There was none which makes me believe that the kinesin I have just doesn't want to stick to beta casein. This is a new phenomenon.
Returning to slide #2 shows that there is still a small amount of motility going on on the slide.
Now I don't know if the reason this is occurring is due to something I did differently when preparing the flow cell of not. I'm going to make a new flow cell not from my stock to see if this is the case.
Third attempt
So I made a fresh flow cell and still I couldn't get it to work. The reason why I made a new one is because when I made the stock batch that I have now, I cleaned the slides when previously, I didn't. I used the new aliquot of kinesin and still used the hot plate at 30C. No motility.
This is exceptionally odd since I know I was able to get this to work 6 months ago and I know I filtered the beta casein PEM solution just like I did this time around. I just don't get it. The whole casein passivation works.
Alpha casein
I'm not sure what is going on with beta casein surface passivation. It just seems to have stopped working for me and I have no clue as to why. It may be because of the kinesin but I'm not sure. At any rate, I'm going to try the alpha casein passivation now.
I don't know if this is an issue but I did freeze all my casein solutions in the -80C freezer. The beta casein didn't work and now I'm going to try the alpha casein. If it doesn't work, then I'm going to try the whole casein again from the freezer. If that doesn't work then I know it's the freezer's fault. However, if the alpha casein works then I think there is something wrong with my kinesin.
First attempt
I see no motility using an alpha casein passivation. Again, I'm going to wait a bit to see if anything happens but it doesn't look good.
Here's what I know so far:
- Cleaned slides are not the issue.
- It's not just the beta casein.
The following could be issues.
- The slide warmer.
- The freezer.
- Filtering.
- It's the kinesin.
If the freezer is the cause, then the whole casein should not work. Although there could be big differences between the constituents of casein and whole casein and how they behave under extreme temperatures.
I know it isn't the slide warmer since I've tried that already and still I saw no motility. I guess I'll try the whole casein from the freezer now.
Whole casein
So if this works, then I still won't know if it's the freezer or if it's the filtering. This will tell me if my kinesin is good or not and I suspect that it is since I was able to get good data yesterday. But, if it doesn't, then it's the freezing or filtering.
I also noticed that there is a lot of moisture in my bench top cooler. This may be causing contamination and I think the moisture came from the power outage recently. Not sure if this is an issue but it's probably a good idea for me to dry the beads out at any rate.
First attempt
It's working. I'm going to take a run of data and add it to yesterday's. So my kinesin is fine.
It would appear that freezing whole casein at -80C is okay as well as filtering it though a 0.2um syringe filter. I am 90% sure that in my earlier casein mixtures I passed them through a 0.2um syringe filter as well so I think it is the freezing that is causing the issue.
I wanted to freeze my casein solutions because I thought it would prolong their shelf life. There is a good thing out of all of this and that is that the assay I ran yesterday used whole casein as the passivation but, since my whole casein was prepared that day, I didn't put the aliquot I used in the freezer. If there is any differences in the speed at which microtubules glide at in this assay from yesterday, I know with better certainty that it's the freezing causing my issues.
I'd also like to point out that I'm seeing lots of very tiny microtubules gliding. This is an excellent indication that my kinesin is good.
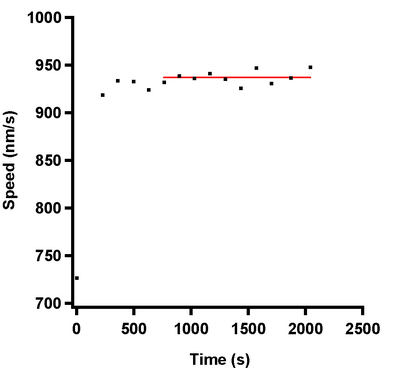
Andy Maloney 23:32, 28 July 2010 (EDT): The results are below.
Nothing odd going on here with the whole casein that was frozen in the -80C freezer. Yesterday's average was 925nm/s and today's average is 937nm/s. I'm assuming that the freezer must be causing the issues with the casein constituents not working as a passivator. I'm going to try tomorrow to make some beta casein without putting it in the freezer and see if I get motility. If I don't, then I'm abandoning this totally and I'm going to exclusively use the whole casein for all of my experiments with heavy waters.
Bench top cooler
I have an unholy fear of contamination now thanks to a certain someone. This is why I evaporated all the moisture from my cooler. It has to cool down now and allows me time to analyze the data I just took to see if there is a speed difference between frozen and unfrozen W-PEM. It also gives me some time to figure out a way to stop moisture from collecting in my cooler.